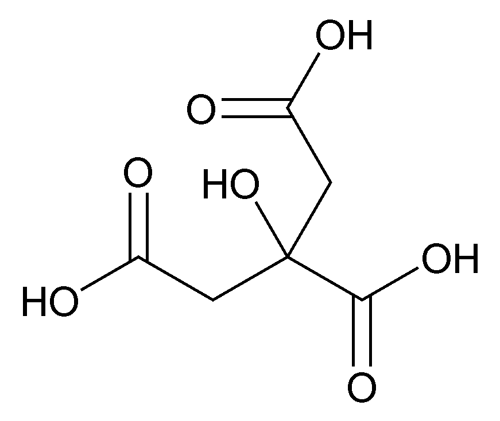
Anhydrous Citric Acid
DEFINITION
Anhydrous Citric Acid contains NLT 99.5% and NMT 100.5% of C6H8O7, calculated on the anhydrous basis.
IDENTIFICATION
• Infrared Absorption  197K
197K :
Dry the substance to be examined at 105
:
Dry the substance to be examined at 105 for 2 h.
for 2 h.
ASSAY
• Procedure
Sample:
0.550 g of Anhydrous Citric Acid; record weight accurately.
Analysis:
Dissolve the Sample in 50 mL of water. Add 0.5 mL of phenolphthalein TS. Titrate with 1 N sodium hydroxide VS. Each mL of 1 N sodium hydroxide is equivalent to 64.03 mg of C6H8O7.
Acceptance criteria:
99.5%–100.5% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%, determined on 1.0 g
:
NMT 0.1%, determined on 1.0 g
• Heavy Metals  231
231 :
NMT 10 ppm
:
NMT 10 ppm
• Sulfate
Standard sulfate solution A:
1.81 mg/mL of potassium sulfate in 30% alcohol. Immediately before use, transfer 10.0 mL of this solution to a 1000-mL volumetric flask, dilute with 30% alcohol to volume, and mix. This solution contains 10 µg/mL of sulfate.
Standard sulfate solution B:
1.81 mg/mL of potassium sulfate in water. Immediately before use, transfer 10.0 mL of this solution to a 1000-mL volumetric flask, dilute with water to volume, and mix. This solution contains 10 µg/mL of sulfate.
Sample stock solution:
66.7 mg/mL of citric acid
Sample solution:
To 4.5 mL of Standard sulfate solution A, add 3 mL of a barium chloride solution (1 in 4), shake, and allow to stand for 1 min. To 2.5 mL of the resulting suspension, add 15 mL of the Sample stock solution and 0.5 mL of 5 N acetic acid, and mix.
Standard solution:
Prepare as directed for the Sample solution, except use 15 mL of Standard sulfate solution B instead of the Sample stock solution.
Analysis
Samples:
Standard solution and Sample solution
Acceptance criteria:
Any turbidity produced in the Sample solution after 5 min standing is not greater than that produced in the Standard solution (0.015%).
• Limit of Aluminum (where it is labeled as intended for use in dialysis)
Standard aluminum solution:
To 352 mg of aluminum potassium sulfate in a 100-mL volumetric flask, add a few mL of water, swirl to dissolve, add 10 mL of diluted sulfuric acid, dilute with water to volume, and mix. Immediately before use, dilute 1.0 mL of this solution with water to 100.0 mL.
pH 6.0 acetate buffer:
Dissolve 50 g of ammonium acetate in 150 mL of water, adjust with glacial acetic acid to a pH of 6.0, dilute with water to 250 mL, and mix.
Standard solution:
Prepare a mixture of 2.0 mL of Standard aluminum solution, 10 mL of pH 6.0 acetate buffer, and 98 mL of water. Extract this mixture as described for the Sample solution, dilute the combined extracts with chloroform to volume, and mix.
Sample solution:
Dissolve 20.0 g of Anhydrous Citric Acid in 100 mL of water, and add 10 mL of pH 6.0 acetate buffer. Extract this solution with successive portions of 20, 20, and 10 mL of a 0.5% solution of 8-hydroxyquinoline in chloroform, combining the chloroform extracts in a 50-mL volumetric flask. Dilute the combined extracts with chloroform to volume, and mix.
Blank solution:
Prepare a mixture of 10 mL of pH 6.0 acetate buffer and 100 mL of water. Extract this mixture as described for Sample solution, dilute the combined extracts with chloroform to volume, and mix.
Fluorometric conditions
Excitation wavelength:
392 nm
Emission wavelength:
518 nm
Analysis
Samples:
Standard solution and Sample solution
Determine the fluorescence intensities of the Samples in a fluorometer set as directed under Fluorometric conditions, using the Blank solution to set the instrument to zero.
Acceptance criteria:
The fluorescence of the Sample solution does not exceed that of the Standard solution (0.2 ppm).
Organic Impurities
• Procedure: Limit of Oxalic Acid
Sample stock solution:
200 mg/mL of Anhydrous Citric Acid in water
Sample solution:
To 4 mL of Sample stock solution add 3 mL of hydrochloric acid and 1 g of granular zinc, boil for 1 min, and allow to stand for 2 min. Transfer the supernatant to a test tube containing 0.25 mL of a phenylhydrazine hydrochloride solution (1 in 100), and heat to boiling. Cool rapidly, transfer to a graduated cylinder, and add an equal volume of hydrochloric acid and 0.25 mL of a potassium ferricyanide solution (1 in 20). Shake, and allow to stand for 30 min.
Standard solution:
Prepare as directed for the Sample solution, except use 4 mL of 0.10 mg/mL oxalic acid solution, equivalent to 0.0714 mg/mL of anhydrous oxalic acid, instead of the Sample stock solution. [Note—Prepare concomitantly with the Sample solution. ]
Analysis
Samples:
Standard solution and Sample solution
Acceptance criteria:
Any pink color produced in the Sample solution is not more intense than that produced in the Standard solution (0.036%).
SPECIFIC TESTS
• Bacterial Endotoxins Test  85
85 :
The level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Anhydrous Citric Acid is used can be met. Where the label states that Anhydrous Citric Acid must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Anhydrous Citric Acid is used can be met.
:
The level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Anhydrous Citric Acid is used can be met. Where the label states that Anhydrous Citric Acid must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Anhydrous Citric Acid is used can be met.
• Clarity of Solution
[Note—The Sample solution is to be compared to Standard suspension A in diffused daylight 5 min after preparation of Standard suspension A. ]
Hydrazine sulfate solution:
10 mg/mL of hydrazine sulfate in water. Allow to stand for 4 to 6 h before use.
Methenamine solution:
Transfer 2.5 g of methenamine to a 100-mL glass-stoppered flask, add 25.0 mL of water, insert the glass stopper, and mix to dissolve.
Primary opalescent suspension:
Transfer 25.0 mL of Hydrazine sulfate solution to the 25.0 mL of Methenamine solution in the 100-mL glass-stoppered flask. Mix, and allow to stand for 24 h. [Note—This suspension is stable for 2 months, provided it is stored in a glass container free from surface defects. The suspension must not adhere to the glass and must be well mixed before use. ]
Opalescence standard:
Dilute 15.0 mL of Primary opalescent suspension with water to 1000 mL. [Note—This suspension should not be used beyond 24 h after preparation. ]
Standard suspension A:
Dilute 5.0 mL of Opalescence standard with water to 100 mL.
Standard suspension B:
Dilute 10.0 mL of Opalescence standard with water to 100 mL.
Sample solution:
200 mg/mL of Anhydrous Citric Acid in water
Analysis
Samples:
Standard suspension A, Standard suspension B, water, and Sample solution
Transfer a sufficient portion of the Sample solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15–25 mm to obtain a depth of 40 mm. Similarly transfer portions of Standard suspension A, Standard suspension B , and water to separate matching test tubes. Compare the Sample solution, Standard suspension A, Standard suspension B, and water in diffused daylight, viewing vertically against a black background (see Spectrophotometry and Light-Scattering  851
851 , Visual Comparison). [Note—The diffusion of light must be such that Standard suspension A can readily be distinguished from water, and that Standard suspension B can readily be distinguished from Standard suspension A. ]
, Visual Comparison). [Note—The diffusion of light must be such that Standard suspension A can readily be distinguished from water, and that Standard suspension B can readily be distinguished from Standard suspension A. ]
Acceptance criteria:
The Sample solution shows the same clarity as that of water.
• Color of Solution
Standard stock solution A:
Ferric chloride CS, cobaltous chloride CS, and dilute hydrochloric acid (10 g/L) (2.4:0.6:7.0)
Standard stock solution B:
Ferric chloride CS, cobaltous chloride CS, cupric sulfate CS, and dilute hydrochloric acid (10 g/L) (2.4:1.0:0.4:6.2)
Standard stock solution C:
Ferric chloride CS, cobaltous chloride CS, and cupric sulfate CS (9.6:0.2:0.2)
[Note—Prepare the Standard solutions immediately before use. ]
Standard solution A:
Dilute 2.5 mL of Standard stock solution A with dilute hydrochloric acid (10 g/L) to 100 mL.
Standard solution B:
Dilute 2.5 mL of Standard stock solution B with dilute hydrochloric acid to (10 g/L) 100 mL.
Standard solution C:
Dilute 0.75 mL of Standard stock solution C with dilute hydrochloric acid (10 g/L) to 100 mL.
Sample solution:
Use the Sample solution prepared as directed in the test for Clarity of Solution.
Analysis
Samples:
Standard solution A, Standard solution B, Standard solution C, water, and Sample solution
Transfer a sufficient portion of the Sample solution to a test tube of colorless, transparent, neutral glass with a flat base and an internal diameter of 15–25 mm to obtain a depth of 40 mm. Similarly transfer portions of Standard solution A, Standard solution B, Standard solution C, and water to separate matching test tubes. Compare the Sample solution, Standard solution A, Standard solution B, Standard solution C, and water in diffused daylight, viewing vertically against a white background (see Spectrophotometry and Light-Scattering  851
851 , Visual Comparison).
, Visual Comparison).
Acceptance criteria:
The Sample solution is not more intensely colored than Standard solution A, B, or C, or water.
• Readily Carbonizable Substances
Sample:
1.0 g of powdered Anhydrous Citric Acid
Analysis:
Transfer the Sample to a 22- × 175-mm test tube previously rinsed with 10 mL of sulfuric acid and allowed to drain for 10 min. Add 10 mL of sulfuric acid, agitate until solution is complete, and immerse in a water bath at 90 ± 1 for 60 ± 0.5 min, keeping the level of the acid below the level of the water during the entire period. Cool the tube in running water, and transfer the acid to a color-comparison tube.
for 60 ± 0.5 min, keeping the level of the acid below the level of the water during the entire period. Cool the tube in running water, and transfer the acid to a color-comparison tube.
Acceptance criteria:
The color of the acid is not darker than that of a similar volume of Matching Fluid K (see Color and Achromicity  631
631 ) in a matching tube, the tubes being observed vertically against a white background.
) in a matching tube, the tubes being observed vertically against a white background.
• Sterility Tests  71
71 :
Where the label states that Anhydrous Citric Acid is sterile, it meets the requirements for Sterility Tests
:
Where the label states that Anhydrous Citric Acid is sterile, it meets the requirements for Sterility Tests  71
71 in the relevant dosage form monograph(s) in which Anhydrous Citric Acid is used.
in the relevant dosage form monograph(s) in which Anhydrous Citric Acid is used.
• Water Determination, Method I  921
921 :
NMT 1.0%
:
NMT 1.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers. No storage requirements specified.
• Labeling:
Where it is intended for use in dialysis solutions, it is so labeled. Where Anhydrous Citric Acid must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of bacterial endotoxins, it is so labeled. Where Anhydrous Citric Acid is sterile, it is so labeled.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2685
Pharmacopeial Forum: Volume No. 34(5) Page 1150