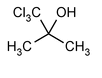Chlorobutanol
(klor'' oh bue' ta nol).
2-Propanol, 1,1,1-trichloro-2-methyl-.
1,1,1-Trichloro-2-methyl-2-propanol
Hemihydrate 186.46
» Chlorobutanol is anhydrous or contains not more than one-half molecule of water of hydration. It contains not less than 98.0 percent and not more than 100.5 percent of C4H7Cl3O, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Label it to indicate whether it is anhydrous or hydrous.
Identification
B:
To 5 mL of a freshly prepared solution (1 in 200) add 1 mL of 1 N sodium hydroxide, then slowly add 3 mL of iodine TS: a yellow precipitate of iodoform, recognizable by its odor, appears.
Reaction—
Shake thoroughly 0.5 g with 25 mL of water: the water remains neutral to litmus.
Water, Method I  921
921 :
not more than 1.0% (anhydrous form) and not more than 6.0% (hydrous form).
:
not more than 1.0% (anhydrous form) and not more than 6.0% (hydrous form).
Chloride—
To a solution of 0.50 g in a mixture of 25 mL of diluted alcohol and 1 mL of nitric acid, add 2 mL of silver nitrate TS: any turbidity produced is not greater than that produced from a control solution containing 0.50 mL of 0.020 N hydrochloric acid in place of the Chlorobutanol (0.07%).
Assay—
Denatured alcohol—
Dilute 100 mL of isopropyl alcohol to 1000 mL with alcohol.
Potassium hydroxide solution—
Transfer 58 g of potassium hydroxide to a 1000-mL volumetric flask. Add 100 mL of water to dissolve, then cool the solution. Dilute with Denatured alcohol to volume, and mix. Prepare fresh just prior to use.
Procedure—
Transfer about 100 mg of Chlorobutanol, accurately weighed, to a glass-stoppered, flat-bottomed boiling flask. Add 50 mL of Potassium hydroxide solution, attach the flask to a reflux condenser, and reflux for 1 hour. Allow the flask to cool while still attached to the condenser, then add 100 mL of water, using a portion of the water to rinse the condenser and its tip. Add 15 mL of nitric acid while stirring. Titrate with 0.1 N silver nitrate VS, determining the endpoint potentiometrically, using a silver-billet combination electrode, consisting of a metallic silver indicator electrode and a double junction reference electrode that allows use of a nonchloride filling solution (such as ammonium nitrate solution) for determining chloride, or equivalent (see Titrimetry  541
541 ). Perform a blank determination, and make any necessary correction. Each mL of 0.1 N silver nitrate is equivalent to 5.915 mg of C4H7Cl3O.
). Perform a blank determination, and make any necessary correction. Each mL of 0.1 N silver nitrate is equivalent to 5.915 mg of C4H7Cl3O.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1758