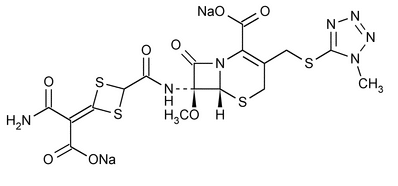
Cefotetan Disodium
(sef'' oh tee' tan dye soe' dee um).
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[[4-(2-amino-1-carboxy-2-oxoethylidene)-1,3-dithietan-2-yl]carbonyl]amino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, disodium salt, [6R-(6
(6R,7S)-4-[[2-Carboxy-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-7-yl]carbamoyl]-1,3-dithietane-D2,
(6R,7S)-7-[4-(Carbamoylcarboxymethylene)-1,3-dithietane-2-carboxamido]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, disodium salt
» Cefotetan Disodium contains the equivalent of not less than 830 µg and not more than 970 µg of cefotetan (C17H17N7O8S4) per mg, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
A:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that of the Standard preparation obtained as directed in the Assay.
B:
It responds to the tests for Sodium  191
191 .
.
pH  791
791 :
between 4.0 and 6.5, in a solution (1 in 10).
:
between 4.0 and 6.5, in a solution (1 in 10).
Water, Method Ic  921
921 :
not more than 2.5%.
:
not more than 2.5%.
Other requirements—
Where the label states that Cefotetan Disodium is sterile, it meets the requirements for Sterility and Bacterial endotoxins under Cefotetan for Injection. Where the label states that Cefotetan Disodium must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Cefotetan for Injection.
Assay—
[note—Protect the Standard preparation, the Resolution solution, and the Assay preparations from light, and use within 2 hours. ]
Mobile phase, Standard preparation, Resolution solution, and Chromatographic system—
Proceed as directed in the Assay under Cefotetan.
Assay preparation—
Transfer about 40 mg of Cefotetan Disodium, accurately weighed, to a 200-mL volumetric flask, add 10 mL of methanol, swirl for several minutes, add 10 mL of acetonitrile, and swirl until dissolved. Dilute with water to volume, and mix.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2561
Pharmacopeial Forum: Volume No. 34(1) Page 86