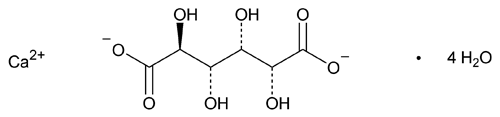
Calcium Saccharate
C6H8CaO8·4H2O 320.26
d-Glucaric acid, calcium salt (1:1) tetrahydrate;
Calcium d-glucarate (1:1), tetrahydrate

 [5793-89-5].
[5793-89-5].
(kal' see um sak' a rate).
C6H8CaO8·4H2O 320.26
d-Glucaric acid, calcium salt (1:1) tetrahydrate;
Calcium d-glucarate (1:1), tetrahydrate
DEFINITION
Calcium Saccharate is the calcium salt of d-saccharic acid. It contains NLT 98.5% and NMT 102.0% of C6H8CaO8·4H2O.
IDENTIFICATION
• A. Identification Tests—General, Calcium  191
191
Sample:
0.2 g
Analysis:
Dissolve the Sample in 10 mL of water by the addition of 2 mL of hydrochloric acid.
Acceptance criteria:
Meets the requirements
• B. Infrared Absorption  197M
197M :
Meets the requirements
:
Meets the requirements
ASSAY
• Procedure
Sample:
600 mg
Blank:
150.0 mL of water
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Direct titration
Titrant:
0.05 M edetate disodium VS
Endpoint detection:
Visual
Analysis:
Dissolve the Sample in 150 mL of water with the aid of a sufficient volume of hydrochloric acid. While stirring, preferably with a magnetic stirrer, add 30 mL of Titrant from a 50-mL buret. Add 15 mL of 1 N sodium hydroxide and 300 mg of hydroxy naphthol blue. Continue the titration to a blue endpoint. Perform a Blank determination and make any necessary corrections.
Calculate the percentage of calcium saccharate (C6H8CaO8·4H2O) in the portion of Calcium Saccharate taken:
Result = {[(VS  VB) × N × F]/W} × 100
VB) × N × F]/W} × 100
| VS | = | = Titrant volume consumed by the Sample (mL) |
| VB | = | = Titrant volume consumed by the Blank (mL) |
| N | = | = actual normality of the Titrant (mEq/mL) |
| F | = | = equivalency factor, 320.2 mg/mEq |
| W | = | = Sample weight (mg) |
Acceptance criteria:
98.5%–102.0%
IMPURITIES
• Chloride and Sulfate, Chloride  221
221
Standard:
0.50 mL of 0.020 N hydrochloric acid
Sample:
0.50 g dissolved in 10 mL of water by the addition of 2 mL of nitric acid
Acceptance criteria:
NMT 0.07%
• Chloride and Sulfate, Sulfate  221
221
Standard:
0.6 mL of 0.020 N sulfuric acid
Sample:
0.50 g dissolved in 10 mL of water by the addition of 2 mL of hydrochloric acid
Acceptance criteria:
NMT 0.12%
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
• Sucrose and Reducing Sugars
Sample:
0.5 g
Analysis:
Dissolve the Sample in 10 mL of water with the addition of 2 mL of hydrochloric acid, and boil the solution for about 2 min. Cool, add 15 mL of sodium carbonate TS, allow to stand for 5 min, and filter. Add 5 mL of the clear filtrate to about 2 mL of alkaline cupric tartrate TS, and boil for 1 min.
Acceptance criteria:
No red precipitate is formed immediately.
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
60 mg/mL in 4.8 N hydrochloric acid that has been allowed to stand for 1 h
Acceptance criteria:
+18.5 to +22.5
to +22.5
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2467
Pharmacopeial Forum: Volume No. 27(6) Page 3261