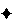 2
K is 5 USP-EU/kg for any route of administration other than intrathecal (for which K is 0.2 USP-EU/kg body weight). For radiopharmaceutical products not administered intrathecally, the endotoxin limit is calculated as 175/V, where V is the maximum recommended dose in mL. For intrathecally administered radiopharmaceuticals, the endotoxin limit is obtained by the formula 14/V. For formulations (usually anticancer products) administered on a per square meter of body surface, the formula is K/M, where K = 2
K is 5 USP-EU/kg for any route of administration other than intrathecal (for which K is 0.2 USP-EU/kg body weight). For radiopharmaceutical products not administered intrathecally, the endotoxin limit is calculated as 175/V, where V is the maximum recommended dose in mL. For intrathecally administered radiopharmaceuticals, the endotoxin limit is obtained by the formula 14/V. For formulations (usually anticancer products) administered on a per square meter of body surface, the formula is K/M, where K =  2.5 USP-EU/kg 2.5 USP-EU/kg (IRA 1-Apr-2011) and M is the (maximum dose/m2/hour × 1.80 m2)/70 Kg. (IRA 1-Apr-2011) and M is the (maximum dose/m2/hour × 1.80 m2)/70 Kg.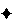
|