INTRODUCTION
There are a variety of presentations for tablets as delivery systems for pharmaceutical agents, such as rapidly disintegrating, slowly disintegrating, eroding, chewable, and lozenge. Each of these presentations places a certain demand on the bonding, structure, and integrity of the compressed matrix. Tablets must be able to withstand the rigors of handling and transportation experienced in the manufacturing plant, in the drug distribution system, and in the field at the hands of the end users (patients/consumers). Manufacturing processes such as coating, packaging, and printing can involve considerable stresses, which the tablets must be able to withstand. For these reasons, the mechanical strength of tablets is of considerable importance and is routinely measured. Tablet strength serves both as a criterion by which to guide product development and as a quality control specification.
One commonly employed test of the ability of tablets to withstand mechanical stresses determines their resistance to chipping and surface abrasion by tumbling them in a rotating cylinder. The percentage weight loss after tumbling is referred to as the friability of the tablets. Standardized methods and equipment for testing friability have been provided in general chapter Tablet Friability  1216
1216 .
.
Another measure of the mechanical integrity of tablets is their breaking force, which is the force required to cause them to fail (i.e., break) in a specific plane. The tablets are generally placed between two platens, one of which moves to apply sufficient force to the tablet to cause fracture. For conventional, round (circular cross-section) tablets, loading occurs across their diameter (sometimes referred to as diametral loading), and fracture occurs in that plane.
The breaking force of tablets is commonly called hardness in the pharmaceutical literature; however, the use of this term is misleading. In material science, the term hardness refers to the resistance of a surface to penetration or indentation by a small probe. The term crushing strength is also frequently used to describe the resistance of tablets to the application of a compressive load. Although this term describes the true nature of the test more accurately than does hardness, it implies that tablets are actually crushed during the test, which often is not the case. Moreover, the term strength in this application can be questioned, because in the physical sciences that term is often used to describe a stress (e.g., tensile strength). Thus, the term breaking force is preferred and will be used in the present discussion.
TABLET BREAKING FORCE DETERMINATIONS
Early measuring devices were typically hand operated. For example, the Monsanto (or Stokes) hardness tester was based on compressing tablets between two jaws via a spring gauge and screw. In the Pfizer hardness tester, the vertically mounted tablet was squeezed in a device that resembled a pair of pliers. In the Strong Cobb hardness tester, the breaking load was applied through the action of a small hydraulic pump that was first operated manually but was later motorized. Problems associated with these devices were related to operator variability in rates of loading and difficulties in proper setup and calibration. Modern testers employ mechanical drives, strain gauge–based load cells for force measurements, and electronic signal processing, and therefore are preferred. However, several important issues must be considered when using them for the analytical determination of breaking force; these are discussed below.
Platens
The platens should be parallel. Their faces should be polished smooth and precision-ground perpendicularly to the direction of movement. Perpendicularity must be preserved during platen movement, and the mechanism should be free of any bending or torsion displacements as the load is applied. The contact faces must be larger than the area of contact with the tablet.
Rate and Uniformity of Loading
Either the rate of platen movement or the rate at which the compressive force is applied (i.e., the loading rate) should be constant. Maintaining a constant loading rate avoids the rapid buildup of compressive loads, which may lead to uncontrolled crushing or shear failure and greater variability in the measured breaking force. However, constant loading rate measurements may be too slow for real time monitoring of tablet production.
The rate at which the compressive load is applied can significantly affect results, because time-dependent processes may be involved in tablet failure (1). How a tablet matrix responds to differences in the loading rate depends on the mechanism of failure. At low strain rates, some materials may fail in a ductile manner, but brittle failure is more likely at faster strain rates. The transition from ductile to brittle failure is accompanied by an increase in the breaking force. Devices that simply crush tablets may produce deceptively reproducible data because they lack sensitivity.
The test must be run consistently with equipment which has been routinely calibrated. Changing from testing units of different designs or from different manufacturers will require comparison of data to ensure that the two units are subjecting the dosage form to similar stress in a similar manner. Currently available equipment provides a constant loading rate of 20 newtons (N) or less per second or a constant platen movement of 3.5 mm or less per second. Controlled and consistent breaking is an important test procedure attribute. To ensure comparability of results, testing must occur under identical conditions of loading rate or platen movement rate. Since there are certain advantages to each system of load application, both are found in practice. Because the particular testing situation and the type of tablet matrix being evaluated will pose different constraints, there is also no basis to declare an absolute preference for one system over the other. This general chapter proposes consideration of both approaches.
The different methods may lead to numerically different results for a particular tablet sample, requiring that the rate of load application or displacement must be specified along with the determined breaking force.
Dependence of Breaking Force on Tablet Geometry and Mass
Measurements of breaking force do not take into account the dimensions or shape of the tablet. Thicker tablets of the same material compressed under conditions identical to those of thinner tablets, with the same tooling shape and to the same peak force, will require greater breaking forces. Tablet orientation and failure should occur in a manner consistent with those used during the development of the dosage form. For direct comparisons (i.e., without any normalizations of the data), breaking force measurements should be performed on tablets having the same dimensions, geometry, and consistent orientation in test equipment.
Tablet Orientation
Tablet orientation in diametral compression of round tablets without any scoring is unequivocal. That is, the tablet is placed between the platens so that compression occurs across a diameter. However, tablets with a unique or complex shape may have no obvious orientation for breaking force determination. Because the breaking force may depend on the tablet's orientation in the tester, to ensure comparability of results, it is best to settle on a standard orientation, preferably one that is most readily and easily reproduced by operators. In general, tablets are tested either across the diameter or parallel to the longest axis. Scored tablets have two orientation possibilities. When they are oriented with their scores perpendicular to the platen faces, the likelihood that tensile failure will occur along the scored line increases. This provides information about the strength of the matrix at the weakest point in the structure. When scored tablets are oriented with their scores parallel to the platen faces, more general information about the strength of the matrix is derived.
Capsule-shaped tablets or scored tablets may best be broken in a three-point flexure test (2). A fitting, which is either installed on the platens or substituted for the platens, supports the tablet at its ends and permits the breaking load to be applied to the opposite face at the unsupported midpoint of the tablet. The fittings are often available from the same source that supplies the hardness tester.
Units, Resolution, and Calibration
Modern breaking force testers are usually calibrated in kiloponds or newtons. The relationship between these units of force (3) is 1 kilopond (kp) = 1 kilogram-force (kgf) = 9.80 N. The test results should be expressed in standard units of force which facilitate communication. Some breaking force testers also will provide a scale in Strong Cobb units (SCU), a carryover from the days when Strong Cobb hardness testers were in common usage. The conversion between SCU and N or kp must be viewed with caution, because the SCU is derived from a hydraulic device and is a pressure.
Generally, contemporary breaking force testers use modern electronic designs with digital readouts. Some units also have an integral printer or may be interfaced with a printer. Breaking forces should be readable to within 1 N.
Breaking force testers should be calibrated periodically. The force sensor as well as the mechanics of the apparatus needs to be considered. For the force sensor, the complete measuring range (or, at a minimum, the range used for measuring the test sample) should be calibrated to a precision of 1 N, using either the static or dynamic method. Static calibration generally employs traceable counterweights; at least three different points are checked to assess linearity. Dynamic calibration makes use of a traceable reference-load cell that is compressed between the platens. The functional calibration of a breaking force test apparatus should also confirm that the velocity and the constancy of velocity for load application or displacement are within prescribed tolerances throughout the range of platen movement.
Sample Size
In order to achieve sufficient statistical precision for the determination of average breaking force, a minimum of 6 tablet samples should be tested. The average breaking force alone may be adequate to fulfill the purpose of process or product quality control. In cases where breaking force may be particularly critical, the average plus individual breaking force values should be accessible.
TENSILE STRENGTH
The measurement of tensile strengths provides a more fundamental measure of the mechanical strength of the compacted material and takes into account the geometry of the tablet. If tablets fail in tension, the breaking force can be used to calculate the tensile strength. Unfortunately, this is practical only for simple shapes. If flat-faced round tablets (right circular cylinders) fail in tension, as indicated by a clean split into halves under diametral compression, the breaking force may be used to compute the tensile strength from the following equation (4), which applies only to cylindrical tablets:
in which 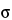 X is the tensile strength, F is the breaking force, D is the tablet diameter, and H is the tablet thickness. Because only tablets that fail in tension are counted, the data are based on tablets that fail in a consistent way. Thus, reproducibility of data should be enhanced when compared to conventional breaking-strength testing. Moreover, the data will be normalized with respect to tablet dimensions, because both diameter and thickness are included in the equation. The derivation of this equation may be found in standard texts (5, 6); it is based on elastic theory and the following assumptions:
X is the tensile strength, F is the breaking force, D is the tablet diameter, and H is the tablet thickness. Because only tablets that fail in tension are counted, the data are based on tablets that fail in a consistent way. Thus, reproducibility of data should be enhanced when compared to conventional breaking-strength testing. Moreover, the data will be normalized with respect to tablet dimensions, because both diameter and thickness are included in the equation. The derivation of this equation may be found in standard texts (5, 6); it is based on elastic theory and the following assumptions:
- The tablet is an isotropic body
- Hooke's law is obeyed
- The modulus of elasticity in compression and in tension is the same
- Ideal point loading occurs
The derivation has been extended to convex-faced tablets (7, 8):
where 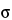 X is the tensile strength, F is the breaking force, D is the tablet diameter, H is the tablet thickness, and W is the central cylinder thickness (tablet wall height).
X is the tensile strength, F is the breaking force, D is the tablet diameter, H is the tablet thickness, and W is the central cylinder thickness (tablet wall height).
The slow and constant loading rate of modern motorized break force testers encourages tensile failure. However, ideal point loading may not occur, because of crushing and the induction of shear failure at the interface with the surface of the platens. The addition of padding to the platens helps prevent shear at contact points and promotes true tensile failure. On that basis, padding is strongly recommended when highly precise measurements are needed. Padding should be relatively thin so that any deviation from the assumption of true point-source force application will not be large. The padding should also collapse very easily so that its deformation does not become part of the force measured by the test apparatus. In more routine settings involving measurements on a large number of samples, the addition of padding could contribute to inaccuracies in measurement as powder from previously tested samples becomes embedded in the collapsible matrix and thereby alters its properties. Unless provisions for frequent and routine replacement of the padding are made, it can be considered acceptable to ignore the use of padding material to maintain constancy of the test conditions.
Bending or flexure of tablets is another option for determining the tensile strength of tablets. Under ideal loading conditions, a breaking load applied to the unsupported midpoint of one face will result in the generation of pure tensile stress in the opposite face. If the tablets are right circular cylinders and are subjected to three-point flexure, the tensile strength may be estimated using the following equation (9):
in which L is the distance between supports, and the other terms are as defined above. The assumptions are the same as those for calculating tensile strength from diametral compression. However, tensile strengths determined by flexure and diametral compression may not agree, because of likely nonideal loading and the induction of shear failure during testing.
REFERENCES
- Davies, P.N.; Newton, J.M. In Pharmaceutical Powder Compaction Technology; Alderbom, G., Nystrom, C., Eds.; Marcel Dekker: New York, 1995; Chapter 7.
- Gold, G.; Duvall, R.N.; Palermo, B.T. New instrumentation for determining flexure breaking strength of capsule-shaped tablets. J. Pharm. Sci. 1980, 69(4), 384–386.
- Diem, K., Ed. Documenta Geigy, Scientific Tables, 6th ed.; Geigy Pharmaceuticals: Ardsley, New York, 1969; 214.
- Fell, J.T.; Newton, J.M. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59(5), 688–691.
- Tomoshenko, S. Theory of Elasticity; McGraw-Hill: New York, 1934; 82–85, 104–109.
- Frocht, M.M. Photoelasticity; John Wiley & Sons: New York, 1948; 2, 32–39, 118–129.
- Stanley, P.; Newton, J.M. The tensile fracture stress of capsule-shaped tablets. J. Pharm. Pharmacol. 1980, 32(12), 852–854.
- Pitt, K.G.; Newton, J.M.; Stanley, P. Tensile fracture of doubly-convex cylindrical discs under diametral loading. J. Mater. Sci. 1988, 23, 2723–2728.
- Pitt, K.G.; Newton, J.M.; Richardson, R.; Stanley, P. The material tensile strength of convex-faced aspirin tablets. J. Pharm. Pharmacol. 1989, 41, 289–292.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison 1-301-816-8106 |
(GCDF2010) General Chapters - Dosage Forms |
USP35–NF30 Page 868
Pharmacopeial Forum: Volume No. 31(6) Page 1695