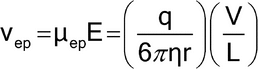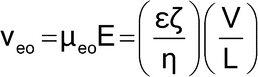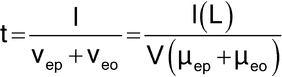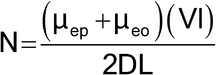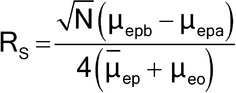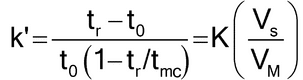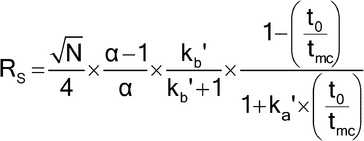This chapter provides guidance and procedures used for characterization of biotechnology-derived articles by capillary electrophoresis. This chapter is harmonized with the corresponding chapter in JP and EP. Other characterization tests, also harmonized, are shown in Biotechnology-Derived Articles—Amino Acid Analysis  1052
1052 , Biotechnology-Derived Articles—Isoelectric Focusing
, Biotechnology-Derived Articles—Isoelectric Focusing  1054
1054 , Biotechnology-Derived Articles—Peptide Mapping
, Biotechnology-Derived Articles—Peptide Mapping  1055
1055 , Biotechnology-Derived Articles—Polyacrylamide Gel Electrophoresis
, Biotechnology-Derived Articles—Polyacrylamide Gel Electrophoresis  1056
1056 , and Biotechnology-Derived Articles—Total Protein Assay
, and Biotechnology-Derived Articles—Total Protein Assay  1057
1057 .
.
INTRODUCTION
Capillary electrophoresis is a physical method of analysis based on the migration, inside a capillary, of charged analytes dissolved in an electrolyte solution under the influence of a direct-current electric field. In this section we are describing four capillary electrophoresis methods: Capillary Zone Electrophoresis, Capillary Gel Electrophoresis, Capillary Isoelectric Focusing, and Micellar Electrokinetic Chromatography.
GENERAL PRINCIPLES
The migration velocity of the analyte under an electric field of intensity E is determined by the electrophoretic mobility of the analyte and the electroosmotic mobility of the buffer inside the capillary. The electrophoretic mobility of a solute (µep) depends on the characteristics of the solute (electrical charge, molecular size, and shape) and the characteristics of the buffer in which the migration takes place (type and ionic strength of the electrolyte, pH, viscosity, and additives). The electrophoretic velocity (vep) of a solute, assuming a spherical shape, is as follows:
in which q is the effective charge of the solute; 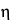 is the viscosity of the electrolyte solution; r is the Stoke's radius of the solute; V is the applied voltage; and L is the total length of the capillary.
is the viscosity of the electrolyte solution; r is the Stoke's radius of the solute; V is the applied voltage; and L is the total length of the capillary.
When an electric field is applied through the capillary filled with buffer, a flow of solvent, called electroosmotic flow, is generated inside the capillary. Its velocity depends on the electroosmotic mobility (µeo), which in turn depends on the charge density on the capillary internal wall and the buffer characteristics. The electroosmotic velocity (veo) is given by the equation:
in which 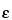 is the dielectric constant of the buffer;
is the dielectric constant of the buffer; 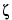 is the zeta potential of the capillary surface; and the other terms are as defined above.
is the zeta potential of the capillary surface; and the other terms are as defined above.
The velocity of the solute (v) is given by the equation:
v = vep + veo
The electrophoretic mobility of the analyte and the electroosmotic mobility may act in the same direction or in opposite directions, depending on the charge of the solute. In normal capillary electrophoresis, anions will migrate in the opposite direction to the electroosmotic flow and their velocities will be smaller than the electroosmotic velocity. Cations will migrate in the same direction as the electroosmotic flow and their velocities will be greater than the electroosmotic velocity. Under conditions in which there is a fast electroosmotic velocity with respect to the electrophoretic velocity of the solutes, both cations and anions can be separated in the same run. The time (t) taken by the solute to migrate the distance (l) from the injection end of the capillary to the detection point (capillary effective length) is as follows:
in which the other terms are as defined above.
In general, uncoated fused-silica capillaries above pH 3 have negative charge due to ionized silanol groups in the inner wall. Consequently, the electroosmotic flow is from anode to cathode. The electroosmotic flow must remain constant from run to run to obtain good reproducibility in the migration velocity of the solutes. For some applications, it might be necessary to reduce or suppress the electroosmotic flow by modifying the inner wall of the capillary or by changing the concentration, composition, and/or the pH of the buffer solution.
After the introduction of the sample into the capillary, each analyte ion of the sample migrates within the background electrolyte as an independent zone according to its electrophoretic mobility. Zone dispersion, that is the spreading of each solute band, results from different phenomena. Under ideal conditions, the sole contribution to the solute-zone broadening is molecular diffusion of the solute along the capillary (longitudinal diffusion). In this ideal case, the efficiency of the zone, expressed as the number of theoretical plates (N), is given by:
in which D is the molecular diffusion coefficient of the solute in the buffer.
In practice, other phenomena, such as heat dissipation, sample adsorption onto the capillary wall, mismatched conductivity between sample and buffer, length of the injection plug, detector cell size, and unleveled buffer reservoirs, can also significantly contribute to band dispersion. Separation between two bands (expressed by the resolution, RS) can be obtained by modification of the electrophoretic mobility of the analytes, by the electroosmotic mobility induced in the capillary, and by increasing the efficiency for the band of each analyte as follows:
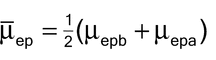
in which µepa and µepb are the electrophoretic mobilities of the two analytes to be separated; µep is the average electrophoretic mobility of the two analytes calculated as:
APPARATUS
An apparatus for capillary electrophoresis is composed of a high voltage controllable direct current power supply; two buffer reservoirs held at the same level and containing specified anodic and cathodic solutions; two electrode assemblies (cathode and anode) immersed in the buffer reservoirs and connected to the power supply; a separation capillary usually made of fused-silica, sometimes with an optical viewing window aligned with the detector, depending on the detector type, with the ends of the capillary placed in the buffer reservoirs and the capillary being filled with a solution specified in a given monograph; a suitable injection system; a detector capable of monitoring the amount of substance of interest passing through a segment of the separation capillary at a given time, generally based on absorption spectrophotometry (UV and visible), fluorimetry, conductimetric, amperometric, or mass spectrometric detection, depending on the specific applications, or even indirect detection to detect non-UV-absorbing and nonfluorescent compounds; a thermostatic system capable of maintaining a constant temperature inside the capillary, recommended to obtain good separation reproducibility; a recorder; and a suitable integrator or a computer.
The definition of the injection process and its automation are critical for precise quantitative analysis. Modes of injection include gravity, pressure or vacuum, or electrokinetic injection. The amount of each sample component introduced electrokinetically depends on its electrophoretic mobility, leading to possible discrimination using this injection mode.
It is expected that the capillary, the buffer solutions, the preconditioning method, the sample solution, and the migration conditions will be specified in the individual monograph. The electrolytic solution employed is filtered to remove particles and degassed to avoid bubble formation that could interfere with the detection system or interrupt the electrical contact in the capillary during the separation run. To achieve reproducible migration time of the solutes, it would be necessary to develop, for each analytical method, a rigorous rinsing routine.
CAPILLARY ZONE ELECTROPHORESIS
Principle
In capillary zone electrophoresis, analytes are separated in a capillary containing only buffer without any anticonvective medium. In this technique, separation takes place because the different components of the sample migrate as discrete bands with different velocities. The velocity of each band depends on the electrophoretic mobility of the solute and the electroosmotic flow on the capillary (see General Principles). Coated capillaries can be used to increase the separation capacity of those substances adsorbing on fused-silica surfaces.
This mode of capillary electrophoresis is appropriate for the analysis of small (MW < 2000) and large (2000 < MW < 100,000) molecules. Due to the high efficiency achieved in capillary zone electrophoresis, separation of molecules having only minute differences in their charge-to-mass ratio can be effected. This separation mode also allows the separation of chiral compounds by addition of chiral selectors to the separation buffer.
Optimization
Optimization of the separation is a complex process where several separation parameters can play a major role. The main factors to be considered in the development of the separations are instrumental and electrolytic solution parameters.
Instrumental Parameters
Voltage—
A Joule heating plot is useful in optimizing the applied voltage and column temperature. The separation time is inversely proportional to applied voltage. However, an increase in the voltage used can cause excessive heat production, giving rise to temperature and, as a result, viscosity gradients in the buffer inside the capillary, which causes band broadening and decreases resolution.
Polarity—
Electrode polarity can be normal (anode at the inlet and cathode at the outlet) and the electroosmotic flow will move toward the cathode. If the electrode polarity is reversed, the electroosmotic flow is away from the outlet and only charged analytes with electroosmotic mobilities greater than the electroosmotic flow will pass to the outlet.
Temperature—
The main effect of temperature is observed on buffer viscosity and electrical conductivity, thus affecting migration velocity. In some cases, an increase in capillary temperature can cause a conformational change of some proteins, modifying their migration time and the efficiency of the separation.
Capillary—
The length and internal diameter of the capillary affects the analysis time, the efficiency of separations, and the load capacity. Increasing both effective length and total length can decrease the electric fields, at a constant voltage, which increases migration time. For a given buffer and electric field, heat dissipation (thus, sample band broadening) depends on the internal diameter of the capillary. The latter also affects the detection limit, depending on the sample volume injected into the capillary and the detection system used.
The adsorption of sample components on the capillary wall limits efficiency; therefore, methods to avoid these interactions should be considered in the development of a separation method. In the specific case of proteins, several strategies have been devised to avoid adsorption on the capillary wall. Some of these strategies (use of extreme pH and adsorption of positively charged buffer additives) only require modification of the buffer composition to prevent protein adsorption. Other strategies include the coating of the internal wall of the capillary with a polymer covalently bonded to the silica that prevents interaction between the proteins and the negatively charged silica surface. For this purpose, ready-to-use capillaries with coatings consisting of neutral-hydrophilic, cationic, and anionic polymers are commercially available.
Electrolytic Solution Parameters
Buffer Type and Concentrations—
Suitable buffers for capillary electrophoresis have an appropriate buffer capacity in the pH range of choice and low mobility to minimize current generation.
To minimize band distortion, it is important to match buffer–ion mobility to solute mobility whenever possible. The type of sample solvent used is important to achieve on-column sample focusing, which increases separation efficiency and improves detection. Also, an increase in buffer concentration at a given pH decreases electroosmotic flow and solute velocity.
Buffer pH—
The pH of the buffer can affect separation by modifying the charge of the analyte or additives and by changing the electroosmotic flow. For protein and peptide separation, a change in the pH of the buffer from above the isoelectric point to below the isoelectric point changes the net charge of the solute from negative to positive. An increase in the buffer pH generally increases the electroosmotic flow.
Organic Solvents—
Organic modifiers, such as methanol, acetonitrile, and others, may be added to the aqueous buffer to increase the solubility of the solute or other additives and/or to affect the ionization degree of the sample components. The addition of these organic modifiers to the buffer generally causes a decrease in the electroosmotic flow.
Additives for Chiral Separations—
To separate optical isomers, a chiral selector is added to the separation buffer. The most commonly used chiral selectors are cyclodextrins, although in some cases crown ethers, certain polysaccharides, or even proteins can be used. Because chiral recognition is governed by the different interactions between the chiral selector and each of the enantiomers, the resolution achieved for the chiral compounds depends largely on the type of chiral selector used. While developing a given separation it may be useful to test cyclodextrins having a different cavity size ( -,
-, 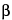 -, or
-, or 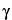 -cyclodextrin) or modified cyclodextrins with neutral (methyl, ethyl, hydroxyalkyl, etc.) or ionizable (aminomethyl, carboxymethyl, sulfobutylether, etc.) moities. When using modified cyclodextrins, batch-to-batch variations in the degree of substitution of the cyclodextrins must be taken into account because it will influence the selectivity. The resolution of chiral separations is also controlled by the concentration of the chiral selector, the composition and pH of the buffer, and the separation temperature. Organic additives, such as methanol or urea, can also affect the resolution of separation.
-cyclodextrin) or modified cyclodextrins with neutral (methyl, ethyl, hydroxyalkyl, etc.) or ionizable (aminomethyl, carboxymethyl, sulfobutylether, etc.) moities. When using modified cyclodextrins, batch-to-batch variations in the degree of substitution of the cyclodextrins must be taken into account because it will influence the selectivity. The resolution of chiral separations is also controlled by the concentration of the chiral selector, the composition and pH of the buffer, and the separation temperature. Organic additives, such as methanol or urea, can also affect the resolution of separation.
CAPILLARY GEL ELECTROPHORESIS
In capillary gel electrophoresis, separation takes place inside a capillary filled with a gel that acts as a molecular sieve. Molecules with similar charge-to-mass ratios are separated according to molecular size because smaller molecules move more freely through the network of the gel and therefore migrate faster than larger molecules. Different biological macromolecules (for example, proteins and DNA fragments), which often have similar charge-to-mass ratios, can thus be separated according to their molecular mass by capillary gel electrophoresis.
Characteristics of Gels
Two types of gels are used in capillary electrophoresis: permanently coated gels and dynamically coated gels. Permanently coated gels are prepared inside the capillary by polymerization of monomers. One example of such a gel is a cross-linked polyacrylamide. This type of gel is usually bonded to the fused-silica wall and cannot be removed without destroying the capillary. For protein analysis under reducing conditions, the separation buffer usually contains sodium dodecyl sulfate, and the sample is denatured by heating in a mixture of sodium dodecyl sulfate and 2-mercaptoethanol or dithiothreitol before injection. When nonreducing conditions are used (for example, analysis of an intact antibody), 2-mercaptoethanol and dithiothreitol are not used. Optimization of separation in a cross-linked gel is obtained by modifying the separation buffer (see Capillary Zone Electrophoresis) and by controlling the gel porosity during the gel preparation. For cross-linked polyacrylamide gels, the porosity can be modified by changing the concentration of acrylamide and/or the ratio of the cross-linker. As a rule, a decrease in the porosity of the gel leads to a decrease in the mobility of the solutes. Due to the rigidity of this type of gel, only electrokinetic injection can be used.
Dynamically coated gels are hydrophilic polymers (i.e., linear polyacrylamide, cellulose derivatives, dextran, etc.) which can be dissolved in aqueous separation buffers, giving rise to a separation medium that also acts as a molecular sieve. These polymeric separation media are easier to prepare than cross-linked polymers. They can be prepared in a vial and filled by pressure in a wall-coated capillary with no electroosmotic flow. Replacing the gel before every injection generally improves the separation reproducibility. The porosity of the dynamically coated gels can be increased by using polymers of higher molecular mass (at a given polymer concentration) or by decreasing the polymer concentration (for a given polymer molecular mass). A decrease in gel porosity leads to a decrease in the mobility of the solute for the same buffer. Both hydrodynamic and electrokinetic injection techniques can be used because the dissolution of these polymers in the buffer gives low viscosity solutions.
CAPILLARY ISOELECTRIC FOCUSING
Principle
In isoelectric focusing the molecules migrate under the influence of the electric field, so long as they are charged, in a pH gradient generated by ampholytes having pI values in a wide range (polyaminocarboxylic acids), dissolved in the separation buffer.
The three basic steps in capillary isoelectric focusing are loading, focusing, and mobilization.
loading
Two methods may be employed.
Loading in One Step—
The sample is mixed with ampholytes and introduced into the capillary by pressure or vacuum.
Sequential Loading—
A leading buffer, then the ampholytes, then the sample mixed with ampholytes, again ampholytes alone, and finally the terminating buffer are introduced into the capillary. The volume of the sample must be small enough so as not to modify the pH gradient.
focusing
When the voltage is applied, ampholytes migrate toward the cathode or the anode according to their net charge, creating the pH gradient from anode (lower pH) to cathode (higher pH). During this step the components to be separated migrate until they reach a pH corresponding to their isoelectric point, and the current drops to very low values.
mobilization
If mobilization is required for detection, use one of the following methods. Three methods are available.
Method 1—
Mobilization is accomplished during Focusing, under the influence of the electroosmotic flow when this flow is small enough to allow the focusing of the components.
Method 2—
Mobilization is accomplished by application of positive pressure after Focusing.
Method 3—
Mobilization is achieved after Focusing, by adding salts to the cathode reservoir or the anode reservoir, depending on the direction chosen for mobilization, in order to alter the pH in the capillary when the voltage is applied. As the pH is changed, the proteins and ampholytes are mobilized in the direction of the reservoir, which contains added salts, and pass the detector.
The separation achieved is expressed as DpI and depends on the pH gradient (dpH/dx), the number of ampholytes having different pI values, the molecular diffusion coefficient (D), the intensity of the electric field (E), and the variation of the electrophoretic mobility of the analyte with the pH ( dµ/dpH):
dµ/dpH):
Optimization
The major parameters that need to be considered in the development of separations are the following:
Voltage—
The use of high fields from 300 V/cm to 1,000 V/cm during Focusing.
Capillary—
Depending on the Mobilization strategy selected (see above), the electroosmotic flow must be reduced or suppressed. Coated capillaries tend to reduce the electroosmotic flow.
Solutions—
The anode buffer reservoir is filled with a solution of a lower pH than the pI of the most acidic ampholyte, and the cathode reservoir is filled with a solution with a higher pH than the pI of the most basic ampholyte. Phosphoric acid for the anode and sodium hydroxide for the cathode are frequently used.
Addition of a polymer, like methylcellulose, in the ampholyte solution tends to suppress convective forces (if any) and electroosmotic flow by increasing the viscosity. Commercial ampholytes covering many pH ranges are available and may also be mixed to obtain an expanded pH range. Broad pH ranges are used to estimate the isoelectric point (pI), whereas narrower ranges are employed to improve accuracy. Calibration can be made by correlating migration time with the isoelectric point of a series of standard protein markers. During Focusing, precipitation of proteins at their isoelectric point can be prevented, if necessary, using buffer additives such as glycerol, surfactants, urea, or zwitterionic buffers. However, depending on the concentration, urea can denature proteins.
MICELLAR ELECTROKINETIC CHROMATOGRAPHY (MEKC)
Principle
Separation takes place in an electrolytic solution that contains a surfactant at a concentration above the critical micellar concentration (cmc). The solute molecules are distributed between the aqueous buffer and the pseudo-stationary phase composed by the micelles according to the solute’s partition coefficient. The technique can be considered as a hybrid of electrophoresis and chromatography. It is a technique that can be used for the separation of both neutral and charged solutes maintaining the efficiency, speed, and instrumental suitability of capillary electrophoresis. One of the most widely used surfactants in MEKC is the anionic surfactant, sodium dodecyl sulfate, although other surfactants, such as cationic surfactant cetyl trimethyl ammonium salts, have also been used.
The separation mechanism is as follows. At neutral and alkaline pH, a strong electroosmotic flow is generated and moves the separation buffer ions in the direction of the cathode. If sodium dodecyl sulfate is used as surfactant, the electrophoretic migration of the anionic micelle is in the opposite direction, towards the anode. As a result, the overall micelle migration velocity is slowed compared to the bulk flow of the electrolytic solution. In the case of neutral solutes, because the analyte can partition between the micelle and the aqueous buffer and has no electrophoretic mobility, the analyte migration velocity will depend only on the partition coefficient between the micelle and the aqueous buffer. In the electropherogram, the peaks corresponding to each uncharged solute are always between that of the electroosmotic flow marker and that of the micelle; and the time elapsed between these two peaks is called the separation window. For electrically charged solutes, the migration velocity depends on both the partition coefficient of the solute between the micelle and the aqueous buffer and on the electrophoretic mobility of the solute in the absence of micelles.
Because the mechanism in MEKC of neutral and weakly ionized solutes is essentially chromatographic, migration of the solute and resolution can be rationalized in terms of the retention factor of the solute (k¢), also referred to as mass distribution ratio (Dm), which is the ratio between the number of moles of solute in the micelle to those in the mobile phase. For a neutral compound, k¢ is as follows:
in which tr is the migration time of the solute; t0 is the analysis time of the unretained solute obtained by injecting an electroosmotic flow marker that does not enter the micelle (e.g., methanol); tmc is the micelle migration time measured by injecting a micelle marker, such as Sudan III, which migrates continuously associated in the micelle; K is the partition coefficient of the solute; VS is the volume of the micellar phase; and VM is the volume of the mobile phase.
The resolution between two closely-migrating solutes (RS) is as follows:
in which N is the number of theoretical plates for one of the solutes;  is the selectivity; and ka¢ and kb¢ are retention factors for both solutes, respectively (kb¢ > ka¢).
is the selectivity; and ka¢ and kb¢ are retention factors for both solutes, respectively (kb¢ > ka¢).
Similar, but not identical, equations give k¢ and RS values for electrically charged solutes.
Optimization
The main parameters to be considered in the development of separations by MEKC are instrumental and electrolytic solution parameters.
instrumental parameters
Voltage—
Separation time is inversely proportional to applied voltage. However, an increase in voltage can cause excessive heat production that gives rise to temperature gradients and viscosity gradients of the buffer in the cross section of the capillary. This effect can be significant with high conductivity buffers, such as those containing micelles. Poor heat dissipation causes band broadening and decreases resolution.
Temperature—
Variations in capillary temperature affect the partition coefficient of the solute between the buffer and the micelles, the critical micellar concentration, and the viscosity of the buffer. These parameters contribute to the migration time of the solutes. The use of a good cooling system improves the reproducibility of the migration time for the solutes.
Capillary—
As in Capillary Zone Electrophoresis, length and internal diameter of the capillary contribute to analysis time and efficiency of separations. Increasing both effective length and total length can decrease the electrical fields, working at constant voltage, and will increase migration time and improve the separation efficiency. The internal diameter controls heat dissipation, for a given buffer and electrical field, and consequently broadening of the sample band.
electrolytic solution parameters
Surfactant Type and Concentration—
The type of surfactant, as the stationary phase in chromatography, affects the resolution because it modifies separation selectively. The log k¢ of a neutral compound increases linearly with the concentration of surfactant in the mobile phase. When k¢ approaches the value of
resolution in MEKC reaches a maximum. Modifying the concentration of surfactant in the mobile phase changes the resolution.
Buffer pH—
pH does not modify the partition coefficient of nonionized solutes, but it can modify the electroosmotic flow in uncoated capillaries. A decrease in the buffer pH decreases the electroosmotic flow and, therefore, increases the resolution of the neutral solutes in MEKC, resulting in a longer analysis time.
Organic Solvents—
To improve MEKC separation of hydrophobic compounds, organic modifiers (methanol, propanol, acetonitrile, etc.) can be added to the electrolytic solution. The addition of these modifiers generally decreases migration time and selectivity of the separation. The addition of organic modifiers affects critical micellar concentration, thus, a given surfactant concentration can be used only with a certain percentage of organic modifier before the micellization is inhibited or adversely affected, resulting in the absence of micelles and, therefore, the absence of the partition. The dissociation of micelles in the presence of a high content of organic solvent does not always mean that the separation will no longer be possible, because in some cases, the hydrophobic interaction between the ionic surfactant monomer and the neutral solutes forms solvophobic complexes that can be separated electrophoretically.
Additives for Chiral Separations—
For the separation of enantiomers using MEKC, a chiral selector is included in the micellar system, either covalently bound to the surfactant or added to the micellar separation electrolyte. Micelles that have a moiety with chiral discrimination properties include salts, N-dodecanoyl-l-amino acids, bile salts, etc. Chiral resolution can also be achieved using chiral discriminators, such as cyclodextrins, added to the electrolytic solutions that contain micellized achiral surfactants.
Other Additives—
Selectivity can be modified by adding chemicals to the buffer. Addition of several types of cyclodextrins to the buffer is also used to reduce the interaction of hydrophobic solutes with the micelle, increasing the selectivity for this type of compound. The addition of substances able to modify solute-micelle interactions by adsorption on the latter has been used to improve the selectivity of the separations in MEKC. These additives may consist of a second surfactant (ionic or nonionic), which gives rise to mixed micelles or metallic cations that dissolve in the micelle and form coordination complexes with the solutes.
Quantification
Peak areas must be divided by the corresponding migration time to give the corrected area in order to compensate for the shift in migration time from run to run, thus reducing the variation of the response. Dividing the peak areas by migration time will also compensate for the different responses of sample constituents with different migration times. Where an internal standard is used, check that no peak of the substance to be examined is masked by that of the internal standard.
Calculations—
From the values obtained, calculate the content of a component or components being determined. When indicated, the percentage of one (or more) components of the sample to be examined is calculated by determining the corrected area(s) of the peak(s) as a percentage of the total of the corrected areas of all the peaks, excluding those due to solvents or any added reagents (normalization procedure). The use of an automatic integration system (integrator or data acquisition and processing system) is recommended.
SYSTEM SUITABILITY
In order to check the behavior of the capillary electrophoresis system, system suitability parameters are used. The choice of these parameters depends on the mode of capillary electrophoresis used. The parameters include the following: retention factor k¢ used only for Micellar Electrokinetic Chromatography, apparent number of theoretical plates (N), the symmetry factor (AS), and the resolution (RS). Note that in previous sections, the theoretical expressions for N and RS have been described, but more practical equations that allow for the determination of these suitability parameters using the electropherograms are described below.
Apparent Number of Theoretical Plates—
The apparent number of theoretical plates (N) may be calculated from the formula:
N = 5.54 (tR/wh)2
in which tR is the migration time or distance along the baseline between the point of injection and the perpendicular dropped from the maximum of the peak corresponding to the component; and wh is the peak width at half-height.
Resolution—
The resolution (RS) between peaks of similar heights of two components may be calculated from the formula:
RS = 1.18 ( tR2  tR1 )/(wh1 + wh2)
tR1 )/(wh1 + wh2)
tR2 > tR1
in which tR1 and tR2 are the migration times or distances along the baseline between the point of injection and the perpendiculars dropped from the maxima of two adjacent peaks; and wh1 and wh2 are the peak widths at half-height.
When appropriate, the resolution (RS) may also be calculated by measuring the height of the valley (Hv) between two partly resolved peaks in a standard preparation, the height of the smaller peak (Hp), and calculating the peak-to-valley ratio:
p/v = Hp/Hv
Symmetry Factor—
The symmetry factor of a peak (AS) may be calculated using the formula:
As = w0.05/2d
in which w0.05 is the width of the peak at one-twentieth of the peak height; and d is the distance between the perpendicular dropped from the peak maximum and the leading edge of the peak at one-twentieth of the peak height.
Other suitability parameters include tests for area repeatability (standard deviation of areas or of area/migration time) and tests for migration time repeatability (standard deviation of migration time). Migration time repeatability provides a test for the suitability of the capillary washing procedures. An alternative practice to avoid the lack of repeatability of the migration time is to use a migration time relative to an internal standard.
Signal-To-Noise Ratio—
A test for the verification of the signal-to-noise ratio for a standard preparation or the determination of the limit of quantification may also be useful for the determination of related substances. The detection limit and quantification limit correspond to a signal-to-noise ratio of 3 and 10, respectively. The signal-to-noise ratio (S/N) is calculated as follows:
S/N = 2H/h
in which H is the height of the peak corresponding to the component concerned in the electropherogram obtained with the specified reference solution, measured from the maximum of the peak to the extrapolated baseline of the signal observed over a distance equal to twenty times the width at half-height; and h is the range of the background in an electropherogram obtained after injection of a blank, observed over a distance equal to twenty times the width at the half-height of the peak in the electropherogram obtained with the prescribed reference solution and, if possible, situated equally around the place where this peak would be found.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Maura C Kibbey, Ph.D.
Senior Scientific Liaison, Biologics & Biotechnology 1-301-816-6309 |
(GCBA2010) General Chapters - Biological Analysis |
USP35–NF30 Page 573
Pharmacopeial Forum: Volume No. 36(1) Page 225