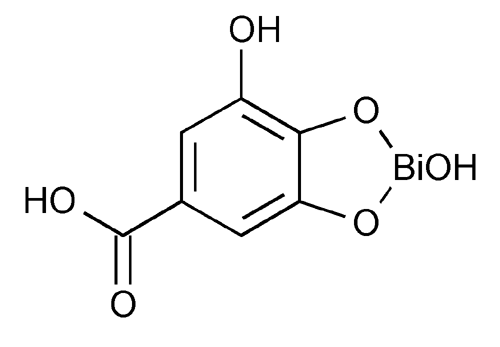
Bismuth Subgallate
» Bismuth Subgallate is a basic salt which, when dried at 105 for 3 hours, contains the equivalent of not less than 52.0 percent and not more than 57.0 percent of Bi2O3.
for 3 hours, contains the equivalent of not less than 52.0 percent and not more than 57.0 percent of Bi2O3.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
A:
When heated to redness, it at first chars, leaving finally a yellow residue. This residue responds to the tests for Bismuth  191
191 .
.
B:
Agitate thoroughly about 100 mg with an excess of hydrogen sulfide TS, filter, and boil the filtrate to expel the dissolved gas. Cool, and add 1 drop of ferric chloride TS: a purplish blue mixture is produced.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 3 hours. It loses not more than 7.0% of its weight.
for 3 hours. It loses not more than 7.0% of its weight.
Limit of nitrate—
Mix about 100 mg with 5 mL of 2 N sulfuric acid and 5 mL of ferrous sulfate TS, filter the mixture, and carefully superimpose the filtrate, without mixing, on 5 mL of sulfuric acid, in a test tube: no reddish brown color appears at the zone of contact of the two liquids.
Arsenic—
Triturate 400 mg with an equal weight of calcium hydroxide, and ignite. Dissolve the residue in 5 mL of 3 N hydrochloric acid: the solution without further treatment meets the requirements of the test for Arsenic  211
211 (7.5 ppm).
(7.5 ppm).
Copper, Lead, and Silver—
Ignite 3 g in a porcelain crucible, cool, and cautiously add, dropwise, just sufficient nitric acid to dissolve the residue upon warming. Evaporate the solution to dryness, again ignite, and cool. Cautiously dissolve the residue in just sufficient nitric acid with the aid of gentle heat, concentrate the solution to about 4 mL, pour it into 100 mL of water, filter, evaporate the filtrate on a steam bath to 20 mL, again filter, and divide this filtrate into portions of 5 mL each. Using these several portions as the test liquid, proceed as directed for Copper, Lead, and Silver under Bismuth Subnitrate. The specified results are obtained.
Limit of alkalies and alkaline earths—
Boil 1.0 g with 20 mL of a mixture of equal volumes of 6 N acetic acid and water, cool, and filter. Precipitate the bismuth from the filtrate by the addition of hydrogen sulfide, boil the mixture, and filter. Add 5 drops of sulfuric acid to the filtrate, evaporate to dryness, and ignite to constant weight: the weight of the residue does not exceed 5 mg (0.5%).
Free gallic acid—
Shake 1.0 g with 20 mL of alcohol for 1 minute, filter and evaporate the filtrate to dryness on a steam bath, and dry the residue at 105 for 1 hour: the weight of the residue does not exceed 5 mg (0.5%).
for 1 hour: the weight of the residue does not exceed 5 mg (0.5%).
Assay—
Dry about 1 g of Bismuth Subgallate at 105 for 3 hours, then weigh accurately and ignite in a porcelain crucible. Allow it to cool and add nitric acid to the residue, dropwise, warming until complete solution has been effected. Evaporate the solution to dryness and carefully ignite the residue to constant weight. From the weight of the residue so obtained, determine the percentage of Bi2O3 in the portion of Bismuth Subgallate taken.
for 3 hours, then weigh accurately and ignite in a porcelain crucible. Allow it to cool and add nitric acid to the residue, dropwise, warming until complete solution has been effected. Evaporate the solution to dryness and carefully ignite the residue to constant weight. From the weight of the residue so obtained, determine the percentage of Bi2O3 in the portion of Bismuth Subgallate taken.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Feiwen Mao, M.S.
Scientist 1-301-816-8320 |
(MDOOD05) Monograph Development-Ophthalmics Oncologics and Dermatologicals |
USP32–NF27 Page 1685