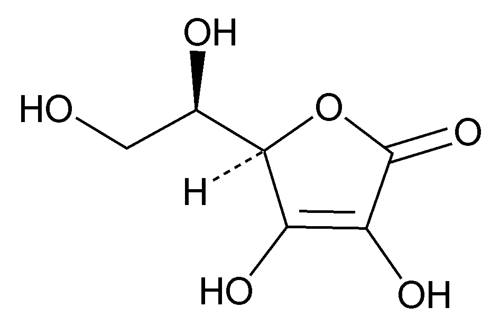
Add the following:
o-Araboascorbic acid.
d-Erythro-hex-2-enoic acid delta-lactone.
Isoascorbic acid, d-isoascorbic acid.
» Erythorbic Acid contains not less than 99.0 percent and not more than the equivalent of 100.5 percent of C6H8O6, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
USP Reference standards  11
11 —
—
USP Erythorbic Acid RS.
USP Erythorbic Acid RS.
Identification—
A:
Infrared Absorption  197K
197K .
.
B:
Add a few drops of sodium nitroferricyanide TS to 2 mL of a solution of Erythorbic Acid and water (1:50), and add 1 mL of 0.1 N sodium hydroxide. A transient blue color immediately appears.
C:
Dissolve about 15 mg of Erythorbic Acid in 15 mL of trichloroacetic acid solution (1:20), add about 200 mg of activated charcoal, and shake the mixture vigorously for 1 minute. Pass through a small fluted filter, refiltering if necessary to obtain a clear filtrate. Add 1 drop of pyrrole to 5 mL of the clear filtrate, agitate the mixture until the pyrrole is dissolved, and heat in a water bath at 50 . A blue color appears.
. A blue color appears.
Specific rotation  781S
781S :
between
:
between  16.5
16.5 and
and  18.0
18.0 .
.
Test solution—
Transfer about 2.5 g of Erythorbic Acid, accurately weighed, into a 25-mL volumetric flask, dissolve in about 20 mL of water, and dilute with water to volume.
Loss on drying  731
731 —
Dry Erythorbic Acid under vacuum over silica gel for 3 hours: it loses not more than 0.4% of its weight.
—
Dry Erythorbic Acid under vacuum over silica gel for 3 hours: it loses not more than 0.4% of its weight.
Residue on ignition  281
281 :
not more than 0.3%.
:
not more than 0.3%.
Limit of lead—
[Note—Select reagents having as low a lead content as practicable, and store all solutions in borosilicate glass containers. Rinse all glassware thoroughly with warm 8 N nitric acid followed by deionized water.]
Standard stock solution—
Dissolve about 160 mg of lead nitrate, accurately weighed, in 100 mL of water containing 1 mL of nitric acid. Dilute with water to 1000 mL, and mix.
Standard solutions—
[note—Prepare these solutions on the day of use.] Transfer 10.0 mL of Standard stock solution to a 100-mL volumetric flask, dilute with water to volume, and mix. Each mL of this solution contains the equivalent of about 10 µg of lead. Dilute accurately measured volumes of the diluted standard solution with water to obtain solutions having known concentrations of about 1 µg, 2 µg, and 5 µg of lead per mL.
Test solution—
Transfer about 10 g of Erythorbic Acid, accurately weighed, to an evaporating dish. Add 5 mL of a 25% sulfuric acid solution, and distribute the sulfuric acid uniformly through the sample. Within a hood, place the dish on a steam bath to evaporate most of the water. Place the dish on a burner, and slowly pre-ash the sample by expelling most of the sulfuric acid. Place the dish in a muffle furnace at 525 , and ash the sample until the residue appears free from carbon. Prepare a blank by ashing 5 mL of a 25% sulfuric acid solution. Cool, and cautiously wash down the inside of each evaporation dish with water. Treat both the sample and the blank as follows. Add 5 mL of 1 N hydrochloric acid. Place each dish on a steam bath, and evaporate to dryness. To each dish add 1.0 mL of 3 N hydrochloric acid and approximately 5 mL of water, and heat briefly on a steam bath to dissolve any residue. Transfer each solution quantitatively to a 10-mL volumetric flask, dilute with water to volume, and mix.
, and ash the sample until the residue appears free from carbon. Prepare a blank by ashing 5 mL of a 25% sulfuric acid solution. Cool, and cautiously wash down the inside of each evaporation dish with water. Treat both the sample and the blank as follows. Add 5 mL of 1 N hydrochloric acid. Place each dish on a steam bath, and evaporate to dryness. To each dish add 1.0 mL of 3 N hydrochloric acid and approximately 5 mL of water, and heat briefly on a steam bath to dissolve any residue. Transfer each solution quantitatively to a 10-mL volumetric flask, dilute with water to volume, and mix.
Procedure—
Using a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with a lead electrodeless discharge lamp, an air–acetylene flame, and a suitable burner head, perform a blank determination with water, following the manufacturer's operating instructions. Concomitantly determine the absorbances of the blank, the Standard solutions, and the Test solution at the lead emission line of 283.3 nm, using a slit width of 0.7 nm. Determine the corrected absorbance values by subtracting the absorbance of the blank from the absorbance of each of the Standard solutions and from the absorbance of the Test solution. Prepare a standard curve by plotting the corrected absorbance values of the Standard solutions versus their corresponding concentration, in µg per mL. From the calibration curve, determine the lead concentration in the Test solution. Calculate the lead content, in µg per g, in the portion of Erythorbic Acid taken by the formula:
) equipped with a lead electrodeless discharge lamp, an air–acetylene flame, and a suitable burner head, perform a blank determination with water, following the manufacturer's operating instructions. Concomitantly determine the absorbances of the blank, the Standard solutions, and the Test solution at the lead emission line of 283.3 nm, using a slit width of 0.7 nm. Determine the corrected absorbance values by subtracting the absorbance of the blank from the absorbance of each of the Standard solutions and from the absorbance of the Test solution. Prepare a standard curve by plotting the corrected absorbance values of the Standard solutions versus their corresponding concentration, in µg per mL. From the calibration curve, determine the lead concentration in the Test solution. Calculate the lead content, in µg per g, in the portion of Erythorbic Acid taken by the formula:
10C/W
in which C is the concentration, in µg per mL, of lead from the standard curve; and W is the weight, in g, of Erythorbic Acid taken: not more than 10 µg per g is found.
Assay—
Dissolve about 400 mg of Erythorbic Acid, accurately weighed, in a mixture of 100 mL of recently boiled and cooled water and 25 mL of 2 N sulfuric acid. Add 3 mL of starch TS, and titrate at once with 0.1 N iodine VS. Each mL of 0.1 N iodine is equivalent to 8.806 mg of C6H8O6. NF27
NF27
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1230
Pharmacopeial Forum: Volume No. 33(6) Page 1246