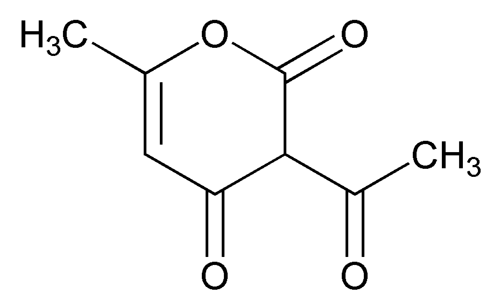
Dehydroacetic Acid
Keto form: 2H-Pyran-2,4(3H)-dione, 3-acetyl-6-methyl-.
3-Acetyl-6-methyl-2H-pyran-2,4(3H)-dione
Enol form: 2H-Pyran-2-one, 3-acetyl-4-hydroxy-6-methyl-.
3-Acetyl-4-hydroxy-6-methyl-2H-pyran-2-one
» Dehydroacetic Acid contains not less than 98.0 percent and not more than 100.5 percent of C8H8O4, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers. No storage requirements specified.
Identification
,
Infrared Absorption  197K
197K .
.
Heavy metals, Method II  231
231 :
not more than 0.001%.
:
not more than 0.001%.
Loss on drying  731
731 :
Dry it at 80
:
Dry it at 80 for 4 hours: it loses not more than 1.0% of its weight.
for 4 hours: it loses not more than 1.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Assay—
Transfer about 500 mg of Dehydroacetic Acid, accurately weighed, into a 250-mL conical flask, dissolve it in 75 mL of neutralized alcohol, add phenolphthalein TS, and titrate with 0.1 N sodium hydroxide VS to a pink endpoint that persists for not less than 30 seconds. Each mL of 0.1 N sodium hydroxide is equivalent to 16.82 mg of C8H8O4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1222
Pharmacopeial Forum: Volume No. 33(4) Page 703