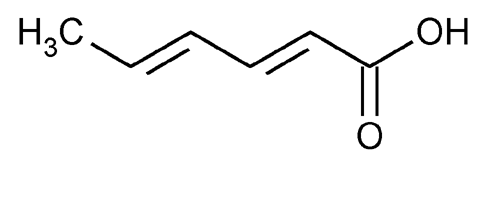
Sorbic Acid
2,4-Hexadienoic acid, (E,E)-; 2,4-Hexadienoic acid.
(E,E)-Sorbic acid; Sorbic acid
» Sorbic Acid contains not less than 99.0 percent and not more than 101.0 percent of C6H8O2, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers, protected from light, and avoid exposure to excessive heat.
Identification—
A:
To a solution of 200 mg in 2 mL of alcohol add a few drops of bromine TS: the color is discharged.
B:
A 1 in 400,000 solution in isopropyl alcohol exhibits an absorbance maximum at 254 ± 2 nm.
Water, Method I  921
921 :
not more than 0.5%.
:
not more than 0.5%.
Residue on ignition  281
281 :
not more than 0.2%.
:
not more than 0.2%.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Assay—
Dissolve about 250 mg of Sorbic Acid, accurately weighed, in a mixture of 50 mL of methanol and 25 mL of water that previously has been neutralized with 0.02 N sodium hydroxide. Add phenolphthalein TS, and titrate with 0.1 N sodium hydroxide VS to the first pink color that persists for at least 30 seconds. Each mL of 0.1 N sodium hydroxide is equivalent to 11.21 mg of C6H8O2.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
USP32–NF27 Page 1348
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.