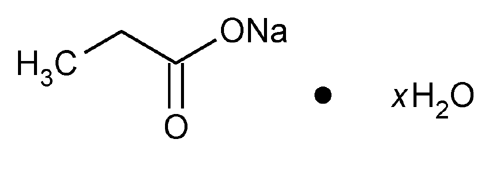
Sodium Propionate
Propanoic acid, sodium salt, hydrate.
Sodium propionate hydrate
Anhydrous 96.06
» Sodium Propionate, dried at 105 for 2 hours, contains not less than 99.0 percent and not more than 100.5 percent of C3H5NaO2.
for 2 hours, contains not less than 99.0 percent and not more than 100.5 percent of C3H5NaO2.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Infrared Absorption  197K
197K , on undried specimen.
, on undried specimen.
B:
A solution (1 in 20) responds to the tests for Sodium  191
191 .
.
Alkalinity—
Dissolve 2.0 g in 20 mL of water, and add phenolphthalein TS: if a pink color is produced, it is discharged by 0.60 mL of 0.10 N sulfuric acid.
Water, Method I  921
921 :
not more than 1.0%.
:
not more than 1.0%.
Heavy metals, Method I  231
231 —
Dissolve 2 g in 1 mL of 1 N acetic acid and sufficient water to make 25 mL: the limit is 0.001%.
—
Dissolve 2 g in 1 mL of 1 N acetic acid and sufficient water to make 25 mL: the limit is 0.001%.
Assay—
Dissolve about 200 mg of Sodium Propionate, previously dried at 105 for 2 hours and accurately weighed, in 50 mL of glacial acetic acid. Add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 9.606 mg of C3H5NaO2.
for 2 hours and accurately weighed, in 50 mL of glacial acetic acid. Add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 9.606 mg of C3H5NaO2.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1345
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.