Sodium Alginate
Alginic acid, sodium salt.
Sodium alginate
» Sodium Alginate is the purified carbohydrate product extracted from brown seaweeds by the use of dilute alkali. It consists chiefly of the sodium salt of Alginic Acid, a polyuronic acid composed of 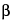 -d-mannuronic acid residues linked so that the carboxyl group of each unit is free while the aldehyde group is shielded by a glycosidic linkage. It contains not less than 90.8 percent and not more than 106.0 percent of sodium alginate of average equivalent weight 222.00, calculated on the dried basis.
-d-mannuronic acid residues linked so that the carboxyl group of each unit is free while the aldehyde group is shielded by a glycosidic linkage. It contains not less than 90.8 percent and not more than 106.0 percent of sodium alginate of average equivalent weight 222.00, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
To 5 mL of a solution (1 in 100) add 1 mL of calcium chloride TS: a voluminous, gelatinous precipitate is formed immediately.
B:
To 10 mL of a solution (1 in 100) add 1 mL of 4 N sulfuric acid: a heavy, gelatinous precipitate is formed.
Microbial enumeration tests  61
61 and Tests for specified microorganisms
and Tests for specified microorganisms  62
62 —
The total bacterial count does not exceed 200 cfu per g, and the tests for Salmonella species and Escherichia coli are negative.
—
The total bacterial count does not exceed 200 cfu per g, and the tests for Salmonella species and Escherichia coli are negative.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 4 hours: it loses not more than 15.0% of its weight.
for 4 hours: it loses not more than 15.0% of its weight.
Total ash  561
561 —
Proceed as directed for Total Ash under Methods of Analysis, carefully igniting about 3 g, accurately weighed, in a tared platinum dish, until the residue is thoroughly carbonized (about 5 minutes), and then igniting in a muffle furnace at a temperature of 800 ± 25
—
Proceed as directed for Total Ash under Methods of Analysis, carefully igniting about 3 g, accurately weighed, in a tared platinum dish, until the residue is thoroughly carbonized (about 5 minutes), and then igniting in a muffle furnace at a temperature of 800 ± 25 until the carbon is completely burned off (approximately 75 minutes): between 18.0% and 27.0% of ash is found, calculated on the dried basis.
until the carbon is completely burned off (approximately 75 minutes): between 18.0% and 27.0% of ash is found, calculated on the dried basis.
Arsenic, Method II  211
211 :
1.5 ppm.
:
1.5 ppm.
Lead  251
251 —
Add 1.0 g to 20 mL of nitric acid in a 250-mL conical flask, mix, and heat carefully until the Sodium Alginate is dissolved. Continue the heating until the volume is reduced to about 7 mL. Cool rapidly to room temperature, transfer to a 100-mL volumetric flask, and dilute with water to volume. A 50.0-mL portion of this solution contains not more than 5 µg of lead (corresponding to not more than 0.001% of Pb), 15 mL of ammonium citrate solution, 3 mL of potassium cyanide solution, and 0.5 mL of hydroxylamine hydrochloride solution being used for the test. After the first dithizone extractions, wash the combined chloroform layers with 5 mL of water, discarding the water layer and continuing in the usual manner by extracting with 20 mL of 0.2 N nitric acid.
—
Add 1.0 g to 20 mL of nitric acid in a 250-mL conical flask, mix, and heat carefully until the Sodium Alginate is dissolved. Continue the heating until the volume is reduced to about 7 mL. Cool rapidly to room temperature, transfer to a 100-mL volumetric flask, and dilute with water to volume. A 50.0-mL portion of this solution contains not more than 5 µg of lead (corresponding to not more than 0.001% of Pb), 15 mL of ammonium citrate solution, 3 mL of potassium cyanide solution, and 0.5 mL of hydroxylamine hydrochloride solution being used for the test. After the first dithizone extractions, wash the combined chloroform layers with 5 mL of water, discarding the water layer and continuing in the usual manner by extracting with 20 mL of 0.2 N nitric acid.
Heavy metals, Method II  231
231 —
Conduct the ignition in a platinum crucible, and use nitric acid in place of sulfuric acid to wet the test specimen: the limit is 0.004%.
—
Conduct the ignition in a platinum crucible, and use nitric acid in place of sulfuric acid to wet the test specimen: the limit is 0.004%.
Assay—
Proceed as directed for Procedure under Alginates Assay  311
311 , using about 250 mg of Sodium Alginate, accurately weighed. Each mL of 0.2500 N sodium hydroxide consumed is equivalent to 27.75 mg of sodium alginate.
, using about 250 mg of Sodium Alginate, accurately weighed. Each mL of 0.2500 N sodium hydroxide consumed is equivalent to 27.75 mg of sodium alginate.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Hong Wang, Ph.D.
Scientist 1-301-816-8351 |
(EM205) Excipient Monographs 2 |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 1339
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.