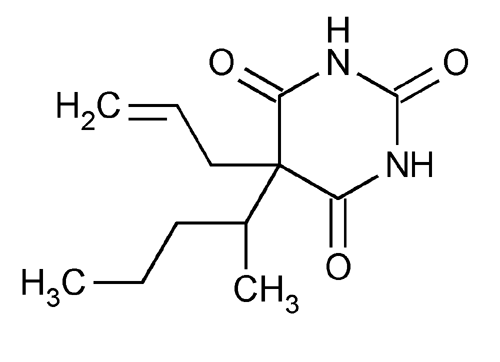
Secobarbital
2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-(1-methylbutyl)-5- (2-propenyl)-.
5-Allyl-5-(1-methylbutyl)barbituric acid
» Secobarbital contains not less than 97.5 percent and not more than 100.5 percent of C12H18N2O3, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification, Infrared Absorption  197M
197M .
.
Loss on drying  731
731 —
Dry it over silica gel for 18 hours: it loses not more than 1.0% of its weight.
—
Dry it over silica gel for 18 hours: it loses not more than 1.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Isomer content—
Dissolve about 300 ± 5 mg in 5 mL of sodium hydroxide solution (1 in 100), add a solution of 300 ± 5 mg of p-nitrobenzyl bromide in 10 mL of alcohol, reflux for 30 minutes, cool, collect the precipitate on a small filter, and wash with water: the precipitate, recrystallized from 25 mL of alcohol and dried at 105 for 30 minutes, melts between 156
for 30 minutes, melts between 156 and 161
and 161 .
.
Assay—
Add about 450 mg of Secobarbital, accurately weighed, to 60 mL of dimethylformamide in a 125-mL conical flask. Add 4 drops of thymol blue TS, and titrate with 0.1 N sodium methoxide VS, using a magnetic stirrer and taking precautions against the absorption of atmospheric carbon dioxide. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N sodium methoxide is equivalent to 23.83 mg of C12H18N2O3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ravi Ravichandran, Ph.D.
Senior Scientist 1-301-816-8330 |
(MDPP05) Monograph Development-Psychiatrics and Psychoactives |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3542
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.