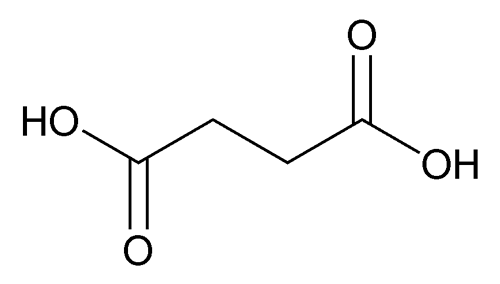
Succinic Acid
» Succinic Acid contains not less than 99.0 percent and not more than 100.5 percent of C4H6O4.
Packaging and storage—
Preserve in a well-closed container. No storage requirements specified.
Identification—
Place a drop of a saturated solution of the sample in a micro test tube, and add a drop of ammonium chloride solution (0.5 in 100) and several mg of zinc powder. Cover the mouth of the tube with a disk of filter paper moistened with a solution in hexane containing p-dimethylaminobenzaldehyde (5 in 100) and trichloroacetic acid (20 in 100). Heat with a small flame for about 1 minute: a pink to red-violet stain appears on the paper.
Melting range  741
741 :
between 185.0 and 190.0.
:
between 185.0 and 190.0.
Residue on ignition  281
281 :
not more than 0.025%.
:
not more than 0.025%.
Heavy metals, Method I  231
231 :
0.002%.
:
0.002%.
Assay—
Dissolve about 250 mg, accurately weighed, in 25 mL of carbon dioxide-free water. Add 2 drops of phenolphthalein TS and titrate with 0.1 N sodium hydroxide VS to the production of a permanent pink color. Each mL of 0.1 N sodium hydroxide is equivalent to 5.905 mg of C4H6O4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
USP32–NF27 Page 1362
Pharmacopeial Forum: Volume No. 32(6) Page 1777