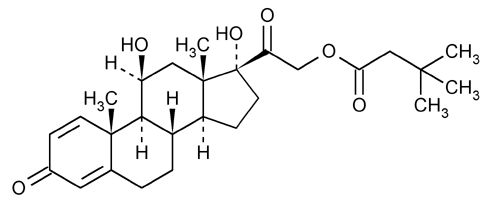
Prednisolone Tebutate
Pregna-1,4-diene-3,20-dione, 11,17-dihydroxy-21-(3,3-dimethyl-1-oxobutyl)oxy-, (11
11
» Prednisolone Tebutate contains not less than 97.0 percent and not more than 103.0 percent of C27H38O6, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers sealed under sterile nitrogen, in a cold place.
Identification—
A:
Dissolve a portion of it in acetone, and evaporate to dryness: the IR absorption spectrum of a mineral oil dispersion of the residue so obtained, previously dried at a pressure not exceeding 5 mm of mercury at 105 for 4 hours, exhibits maxima only at the same wavelengths as that of a similar preparation of USP Prednisolone Tebutate RS.
for 4 hours, exhibits maxima only at the same wavelengths as that of a similar preparation of USP Prednisolone Tebutate RS.
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
20 µg per mL.
Medium:
methanol.
Absorptivities at 242 nm, calculated on the dried basis, do not differ by more than 3.0%.
Loss on drying  731
731 —
Dry it at a pressure not exceeding 5 mm of mercury at 105
—
Dry it at a pressure not exceeding 5 mm of mercury at 105 for 4 hours: it loses not more than 5.0% of its weight.
for 4 hours: it loses not more than 5.0% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Selenium  291
291 :
0.003%, a 200-mg specimen being used.
:
0.003%, a 200-mg specimen being used.
Assay—
Mobile solvent—
Prepare a mixture of isooctane, tetrahydrofuran, and alcohol (89:10:1).
Standard preparation—
Dissolve a suitable quantity of USP Prednisolone Tebutate RS, accurately weighed, in a mixture of tetrahydrofuran and isooctane (1:1) to obtain a solution having a known concentration of about 1 mg per mL.
Assay preparation—
Weigh accurately about 50 mg of Prednisolone Tebutate, transfer to a 50-mL volumetric flask, dilute with tetrahydrofuran and isooctane (1:1) to volume, and mix.
Procedure—
Inject separately 25-µL volumes of the Standard preparation and the Assay preparation into a suitable high-pressure liquid chromatograph equipped with a constant flow pump and a 30-cm × 3.9-mm column that contains packing L3, is operated at 25 , and is equipped with an UV detector capable of monitoring absorption at 254 nm. The flow rate is about 1.0 mL per minute. The retention time for Prednisolone Tebutate is about 30 minutes, but the chromatogram is run for about 45 minutes. Five replicate injections of the Standard preparation show a relative standard deviation of not more than 1.5%. Calculate the quantity, in mg, of C27H38O6 in the portion of Prednisolone Tebutate taken by the formula:
, and is equipped with an UV detector capable of monitoring absorption at 254 nm. The flow rate is about 1.0 mL per minute. The retention time for Prednisolone Tebutate is about 30 minutes, but the chromatogram is run for about 45 minutes. Five replicate injections of the Standard preparation show a relative standard deviation of not more than 1.5%. Calculate the quantity, in mg, of C27H38O6 in the portion of Prednisolone Tebutate taken by the formula:
50C(RU / RS)
in which C is the concentration, in mg per mL, of USP Prednisolone Tebutate RS in the Standard preparation, and RU and RS are the peak areas obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3375