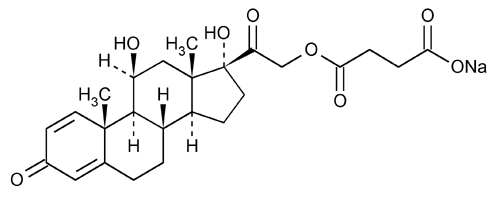
Prednisolone Sodium Succinate for Injection
Pregna-1,4-diene-3,20-dione, 21-(3-carboxy-1-oxopropoxy)-11,17-dihydroxy-, monosodium salt, (11
11
» Prednisolone Sodium Succinate for Injection is sterile prednisolone sodium succinate prepared from Prednisolone Hemisuccinate with the aid of Sodium Hydroxide or Sodium Carbonate. It contains the equivalent of not less than 90.0 percent and not more than 110.0 percent of the labeled amount of prednisolone (C21H28O5). It contains suitable buffers.
Constituted solution—
At the time of use, it meets the requirements for Constituted Solutions under Injections  1
1 .
.
Identification—
Place about 50 mg in a separator, add 20 mL of water and 2 mL of 3 N hydrochloric acid, and extract with 25 mL of chloroform. Filter the extract into a suitable beaker, evaporate on a steam bath to dryness, and dry the residue at 60 for 1 hour: the residue so obtained responds to Identification test A under Prednisolone Hemisuccinate.
for 1 hour: the residue so obtained responds to Identification test A under Prednisolone Hemisuccinate.
Bacterial endotoxins  85
85 —
It contains not more than 5.8 USP Endotoxin Units per mg of prednisolone.
—
It contains not more than 5.8 USP Endotoxin Units per mg of prednisolone.
pH  791
791 :
between 6.7 and 8.0, determined in the solution constituted as directed in the labeling.
:
between 6.7 and 8.0, determined in the solution constituted as directed in the labeling.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 3 hours: it loses not more than 2.0% of its weight.
for 3 hours: it loses not more than 2.0% of its weight.
Particulate matter  788
788 :
meets the requirements under small-volume injections.
:
meets the requirements under small-volume injections.
Other requirements—
It meets the requirements for Sterility Tests  71
71 , Uniformity of Dosage Units
, Uniformity of Dosage Units  905
905 and Labeling under Injections
and Labeling under Injections  1
1 .
.
Assay—
Standard preparation—
Dissolve about 64 mg of USP Prednisolone Hemisuccinate RS, accurately weighed, in about 100 mL of alcohol, add 5.0 mL of water, dilute with alcohol to 200.0 mL, and mix. Dilute 4.0 mL of this solution with alcohol to 100.0 mL, and mix. Pipet 20 mL of the resulting solution into a glass-stoppered, 50-mL conical flask.
Assay preparation—
Transfer an accurately weighed portion of Prednisolone Sodium Succinate for Injection, equivalent to about 50 mg of prednisolone, to a 200-mL volumetric flask, dissolve in 5.0 mL of water, add alcohol to volume, and mix. Dilute 4.0 mL of this mixture with alcohol to 100.0 mL, and mix. Pipet 20 mL of the resulting solution into a glass-stoppered, 50-mL conical flask.
Procedure—
Proceed as directed under Assay for Steroids  351
351 . Calculate the quantity, in mg, of C21H28O5 in the portion of Prednisolone Sodium Succinate for Injection taken by the formula:
. Calculate the quantity, in mg, of C21H28O5 in the portion of Prednisolone Sodium Succinate for Injection taken by the formula:
5C(0.7827)(AU / AS)
in which C is the concentration, in µg per mL, of USP Prednisolone Hemisuccinate RS in the Standard preparation, 0.7827 is the ratio of the molecular weight of prednisolone to that of prednisolone hemisuccinate, and AU and AS are the absorbances of the solutions from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 3375
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.