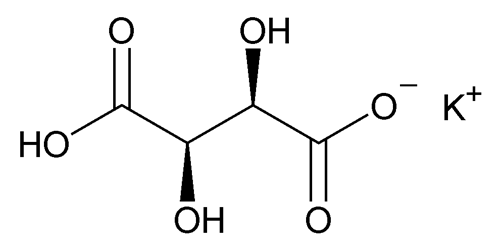
Potassium Bitartrate
Butanedioic acid 2,3-dihydroxy-, [R-(R*,R*)]-, monopotassium salt.
Potassium hydrogen tartrate
» Potassium Bitartrate, dried at 105 for 3 hours, contains not less than 99.0 percent and not more than 101.0 percent of C4H5KO6.
for 3 hours, contains not less than 99.0 percent and not more than 101.0 percent of C4H5KO6.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Ignite it: it leaves a residue that imparts a reddish purple color to a nonluminous flame.
B:
A saturated solution of it yields a yellowish orange precipitate with sodium cobaltinitrite TS.
C:
A solution (1 in 10) responds to the tests for Tartrate  191
191 .
.
Insoluble matter—
Mix 500 mg of it with 3 mL of 6 N ammonium hydroxide: no undissolved residue remains.
Limit of ammonia—
Sodium hypochlorite solution—
Use a commercially available solution that contains between 4.0% and 6.0% of sodium hypochlorite.
Oxidizing solution—
[note—Prepare on the day of use.] Prepare a mixture of alkaline sodium citrate TS and Sodium hypochlorite solution (4:1).
Diluted sodium nitroferricyanide solution—
Prepare a mixture of water and sodium nitroferricyanide TS (10:1).
Standard solution—
Transfer 300 mg of ammonium chloride, previously dried over silica gel for 4 hours, to a 1-L volumetric flask, and dilute with water to volume. This solution contains 100 µg of ammonia per mL. Dilute this solution quantitatively, and stepwise if necessary, with water to obtain a solution having a concentration of 0.25 µg of ammonia per mL.
Test solution—
Transfer 250 mg of Potassium Bitartrate to a 100-mL volumetric flask, and dissolve in and dilute with water to volume. Heat gently to facilitate the dissolution.
Procedure—
[note—Carefully follow the order of addition stated below.] Separately transfer 6.0 mL each of the Standard solution and the Test solution to two color-comparison tubes. To each tube add 0.4 mL of phenol TS, 0.4 mL of Diluted sodium nitroferricyanide solution, and 1.0 mL of Oxidizing solution. Dilute with water to 10 mL, mix, and allow to stand for 1 hour: the color of the Test solution is not darker than the color of the Standard solution (0.01%).
Heavy metals, Method I  231
231 —
Mix 2 g of it with 15 mL of water, and add 6 N ammonium hydroxide dropwise until solution is complete. Add 1 drop of phenolphthalein TS, and add just sufficient 1 N acetic acid dropwise to discharge the pink color. Add 2 mL of 1 N acetic acid, and dilute with water to 25 mL: the limit is 0.002%.
—
Mix 2 g of it with 15 mL of water, and add 6 N ammonium hydroxide dropwise until solution is complete. Add 1 drop of phenolphthalein TS, and add just sufficient 1 N acetic acid dropwise to discharge the pink color. Add 2 mL of 1 N acetic acid, and dilute with water to 25 mL: the limit is 0.002%.
Assay—
Dry about 6 g of Potassium Bitartrate at 105 for 3 hours, allow to cool, and weigh accurately. Dissolve in 100 mL of boiling water, add a few drops of phenolphthalein TS, and titrate with 1 N sodium hydroxide VS to a pink endpoint. Perform a blank determination, and make any necessary correction. Each mL of 1 N sodium hydroxide is equivalent to 188.2 mg of C4H5KO6.
for 3 hours, allow to cool, and weigh accurately. Dissolve in 100 mL of boiling water, add a few drops of phenolphthalein TS, and titrate with 1 N sodium hydroxide VS to a pink endpoint. Perform a blank determination, and make any necessary correction. Each mL of 1 N sodium hydroxide is equivalent to 188.2 mg of C4H5KO6.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
USP32–NF27 Page 3334
Pharmacopeial Forum: Volume No. 34(5) Page 1180