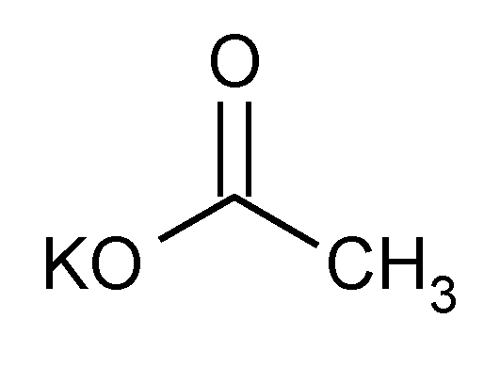
Potassium Acetate
» Potassium Acetate contains not less than 99.0 percent and not more than 100.5 percent of C2H3KO2, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
pH  791
791 :
between 7.5 and 8.5, in a solution (1 in 20).
:
between 7.5 and 8.5, in a solution (1 in 20).
Loss on drying  731
731 —
Dry it at 150
—
Dry it at 150 for 2 hours: it loses not more than 1.0% of its weight.
for 2 hours: it loses not more than 1.0% of its weight.
Heavy metals, Method I  231
231 —
Prepare the Test Preparation as follows. Dissolve 1 g in 10 mL of water, add 3.0 mL of glacial acetic acid, dilute with water to 25 mL, and adjust with glacial acetic acid to a pH between 3.8 and 4.0, measured with a pH meter. Prepare the Monitor Preparation as directed for Test Preparation, 2.0 mL of Standard Lead Solution being added: the limit is 0.002%.
—
Prepare the Test Preparation as follows. Dissolve 1 g in 10 mL of water, add 3.0 mL of glacial acetic acid, dilute with water to 25 mL, and adjust with glacial acetic acid to a pH between 3.8 and 4.0, measured with a pH meter. Prepare the Monitor Preparation as directed for Test Preparation, 2.0 mL of Standard Lead Solution being added: the limit is 0.002%.
Limit of sodium—
Potassium chloride solution—
Dissolve 100 g of potassium chloride in water to make 1000 mL.
Standard solutions—
Transfer 127.1 mg of sodium chloride, previously dried at 105 for 2 hours and accurately weighed, to a 500-mL volumetric flask, add water to volume, and mix. Transfer 10.0 mL of this solution to a 100-mL volumetric flask, dilute with water to volume, and mix. Transfer 2.0, 5.0, and 10.0 mL of this solution to separate 100-mL volumetric flasks, add 10.0 mL of Potassium chloride solution to each flask, dilute with water to volume, and mix. These Standard solutions contain 0.2, 0.5, and 1.0 µg of sodium per mL, respectively. [note—Concentrations of sodium in the Standard solutions may be modified to fit the linear or working range of the atomic absorption spectrophotometer.]
for 2 hours and accurately weighed, to a 500-mL volumetric flask, add water to volume, and mix. Transfer 10.0 mL of this solution to a 100-mL volumetric flask, dilute with water to volume, and mix. Transfer 2.0, 5.0, and 10.0 mL of this solution to separate 100-mL volumetric flasks, add 10.0 mL of Potassium chloride solution to each flask, dilute with water to volume, and mix. These Standard solutions contain 0.2, 0.5, and 1.0 µg of sodium per mL, respectively. [note—Concentrations of sodium in the Standard solutions may be modified to fit the linear or working range of the atomic absorption spectrophotometer.]
Test solution—
Transfer about 0.2 g of Potassium Acetate, accurately weighed, to a 100-mL volumetric flask containing about 50 mL of water, and swirl to dissolve. Add 10.0 mL of Potassium chloride solution, dilute with water to volume, and mix. [note—The concentration of Potassium Acetate in the Test solution may be modified by using a different quantity or by further dilution to bring the absorption response within the range of responses obtained from the Standard solutions.]
Blank solution—
Transfer 10.0 mL of Potassium chloride solution to a 100-mL volumetric flask, dilute with water to volume, and mix.
Procedure—
Concomitantly determine the absorbances of the Standard solutions and the Test solution at the sodium emission line of 589 nm, with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with a sodium hollow-cathode lamp and an oxidizing air–acetylene flame, using the Blank solution to zero the instrument. Plot the absorbances of the Standard solutions versus concentration, in µg per mL, of sodium, and draw the straight line best fitting the plotted points. From the graph so obtained, determine the concentration C, in µg per mL, of sodium in the Test solution. Calculate the percentage of sodium in the portion of Potassium Acetate taken by the formula:
) equipped with a sodium hollow-cathode lamp and an oxidizing air–acetylene flame, using the Blank solution to zero the instrument. Plot the absorbances of the Standard solutions versus concentration, in µg per mL, of sodium, and draw the straight line best fitting the plotted points. From the graph so obtained, determine the concentration C, in µg per mL, of sodium in the Test solution. Calculate the percentage of sodium in the portion of Potassium Acetate taken by the formula:
CD / 10,000W
in which W is the quantity, in g, of Potassium Acetate taken to prepare the Test solution; and D is the extent of dilution of the Test solution: not more than 0.03% of sodium is found.
Assay—
Dissolve about 200 mg of Potassium Acetate, previously dried and accurately weighed, in 25 mL of glacial acetic acid, add 2 drops of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 9.814 mg of C2H3KO2.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Feiwen Mao, M.S.
Scientist 1-301-816-8320 |
(MDOOD05) Monograph Development-Ophthalmics Oncologics and Dermatologicals |
USP32–NF27 Page 3330
Pharmacopeial Forum: Volume No. 28(4) Page 1185