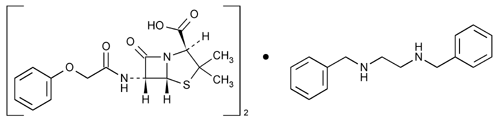
Penicillin V Benzathine
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-6-[(2-phenoxyacetyl)amino-], [2S-(2
(2S,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenoxyacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid compound with N,N¢-dibenzylethylenediamine (2:1)
Tetrahydrate 1013.21
» Penicillin V Benzathine has a potency of not less than 1060 and not more than 1240 Penicillin V Units per mg.
Packaging and storage—
Preserve in tight containers.
Crystallinity  695
695 :
meets the requirements.
:
meets the requirements.
pH  791
791 :
between 4.0 and 6.5, in a suspension containing about 30 mg per mL.
:
between 4.0 and 6.5, in a suspension containing about 30 mg per mL.
Water, Method I  921
921 :
between 5.0% and 8.0%.
:
between 5.0% and 8.0%.
Penicillin V content—
Transfer about 40 mg, accurately weighed, to a 100-mL volumetric flask, add methanol to volume, and mix. Concomitantly determine the absorbances of this solution and of a similarly prepared Standard solution prepared with about 30 mg of USP Penicillin V Potassium RS at the wavelength of maximum absorbance at about 276 nm. Determine the percentage of penicillin V taken by the formula:
P(aU / aS)
in which P is the percentage content of penicillin V in the USP Penicillin V Potassium RS, and aU and aS are the absorptivities of the solution of the specimen and the Standard solution, respectively: between 62.3% and 72.5% is found.
Assay—
Standard preparation—
Prepare as directed for Standard preparation under Iodometric Assay—Antibiotics  425
425 , using USP Penicillin V Potassium RS.
, using USP Penicillin V Potassium RS.
Assay preparations—
Dissolve a quantity of Penicillin V Benzathine in 1.0 N sodium hydroxide to obtain a solution containing 2000 Penicillin V Units per mL. Pipet 2 mL of this solution into a glass-stoppered, 125-mL conical flask, and use as the Assay preparation for Inactivation and titration. Dilute a quantity of Penicillin V Benzathine quantitatively with water to obtain a suspension containing 2000 Penicillin V Units per mL. Pipet 2 mL of this suspension into a glass-stoppered, 125-mL conical flask, and use as the Assay preparation for the Blank determination.
Procedure—
Proceed as directed for Procedure under Iodometric Assay—Antibiotics  425
425 , except in the Inactivation and titration of the specimen to omit the addition of 2.0 mL of 1.0 N sodium hydroxide. Calculate the potency, in Penicillin V Units per mg, in the Penicillin V Benzathine taken by the formula:
, except in the Inactivation and titration of the specimen to omit the addition of 2.0 mL of 1.0 N sodium hydroxide. Calculate the potency, in Penicillin V Units per mg, in the Penicillin V Benzathine taken by the formula:
(F)(B  I) / (2D)
I) / (2D)
in which D is the concentration, in mg per mL, of the Assay preparation for Inactivation and titration on the basis of the weight of Penicillin V Benzathine taken and the extent of dilution.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Ahalya Wise, M.S.
Scientist 1-301-816-8161 |
(MDANT05) Monograph Development-Antibiotics |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 3241