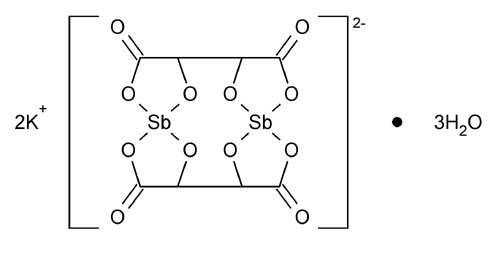
Antimony Potassium Tartrate
Antimonate(2-), bis[µ-[2,3-dihydroxybutanedioato(4-)-O1,O2:O3,O4]]-di-, dipotassium, trihydrate, stereoisomer.
Dipotassium bis[µ-[l-(+)-tartrato(4-)]]diantimonate(2-) trihydrate
Anhydrous 613.82
» Antimony Potassium Tartrate contains not less than 99.0 percent and not more than 103.0 percent of C8H4K2O12Sb2·3H2O.
Packaging and storage—
Preserve in well-closed containers.
Completeness of solution  641
641 :
meets the requirements, using a 750-mg specimen and water as the solvent.
:
meets the requirements, using a 750-mg specimen and water as the solvent.
Identification—
A:
When heated to redness, it chars, emits an odor resembling that of burning sugar, and leaves a blackened residue. This residue has an alkaline reaction, and when a small fragment of it is held in a nonluminous flame, the flame is tinted violet.
B:
In a solution (1 in 20), acidified with hydrochloric acid, hydrogen sulfide TS produces an orange-red precipitate, which is soluble in ammonium sulfide TS and in 1 N sodium hydroxide.
C:
It responds to the test for Tartrate  191
191 .
.
Acidity or alkalinity—
Dissolve 1.0 g in 50 mL of carbon dioxide-free water, and titrate with 0.010 N hydrochloric acid or 0.010 N sodium hydroxide to a pH of 4.5: not more than 2.0 mL is required.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 to constant weight: it loses not more than 2.7% of its weight.
to constant weight: it loses not more than 2.7% of its weight.
Arsenic—
Dissolve 100 mg in 5 mL of hydrochloric acid. Add 10 mL of a recently prepared solution of 20 g of stannous chloride in 30 mL of hydrochloric acid. Mix, transfer to a color-comparison tube, and allow to stand for 30 minutes. Viewed downward over a white surface, the color of the solution appears no deeper than that of a blank to which has been added 15 µg of arsenic (0.015%).
Lead  251
251 :
not more than 0.002%.
:
not more than 0.002%.
Assay—
Dissolve about 500 mg of Antimony Potassium Tartrate, accurately weighed, in 50 mL of water, add 5 g of potassium sodium tartrate, 2 g of sodium borate, and 3 mL of starch TS, and immediately titrate with 0.1 N iodine VS to the production of a persistent blue color. Each mL of 0.1 N iodine is equivalent to 16.70 mg of C8H4K2O12Sb2·3H2O.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientist 1-301-816-8394 |
(MDAA05) Monograph Development-Antivirals and Antimicrobials |
USP32–NF27 Page 1568