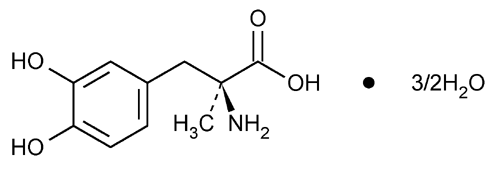
Methyldopa
l-Tyrosine, 3-hydroxy-
l-3-(3,4-Dihydroxyphenyl)-2-methylalanine sesquihydrate
Anhydrous 211.22
» Methyldopa contains not less than 98.0 percent and not more than 101.0 percent of C10H13NO4, calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed, light-resistant containers.
Identification—
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
40 µg per mL.
Medium:
0.1 N hydrochloric acid.
Absorptivities at 280 nm, calculated on the anhydrous basis, do not differ by more than 3.0%.
C:
To 10 mg add 0.15 mL of a solution of ninhydrin in sulfuric acid (1 in 250): a dark purple color is produced within 5 to 10 minutes. Add 0.15 mL of water: the color changes to pale brownish yellow.
Specific rotation  781S
781S :
between
:
between  25
25 and
and  28
28 .
.
Test solution:
44 mg per mL, in a solvent that is a solution of aluminum chloride in water (2 in 3) which previously has been treated with activated charcoal, filtered, and adjusted with 0.25 N sodium hydroxide to a pH of 1.5.
Acidity—
Dissolve 1.0 g in carbon dioxide-free water with the aid of heat, add 1 drop of methyl red TS, and titrate with 0.10 N sodium hydroxide to a yellow endpoint: not more than 0.50 mL is required.
Water, Method I  921
921 :
between 10.0% and 13.0%.
:
between 10.0% and 13.0%.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Limit of 3-O-methylmethyldopa—
Developing solvent—
Mix 65 parts by volume of butyl alcohol, 15 parts by volume of glacial acetic acid, and 25 parts by volume of water. Prepare this mixture fresh.
Chromatographic plate—
Prepare a thin-layer chromatographic plate with a suitable grade of cellulose, 250 µm thick, prewashed with the Developing solvent. Wash the plate by placing it in a tank containing the solvent system and allowing the solvent to rise to the top of the plate. Dry with the aid of a current of dry air.
Spray solution 1—
Dissolve 300 mg of p-nitroaniline in 100 mL of 10 N hydrochloric acid (Solution A). Dissolve 2.5 g of sodium nitrite in 50 mL of water (Solution B). Mix 90 mL of Solution A and 10 mL of Solution B (Spray solution 1). Prepare all solutions fresh, just before spraying.
Spray solution 2—
Dissolve 25 g of sodium carbonate in 100 mL of water, and mix.
Test solution—
Dissolve 100 mg of Methyldopa in methanol, and dilute with methanol to 10.0 mL.
Standard solution—
Dissolve 5.0 mg of USP 3-O-Methylmethyldopa RS in methanol, and dilute with methanol to 50.0 mL to obtain a Standard solution having a known concentration of 100 µg per mL.
Procedure—
Apply 20 µL of the Test solution in two 10-µL increments and 10 µL of the Standard solution to the Chromatographic plate, so that the spots are not larger than 0.5 cm in diameter. Develop the chromatogram using the Developing solvent until the solvent front has moved about 10 cm from the origin. Remove the plate from the chamber, and dry with the aid of a current of dry air until no odor of acetic acid is perceptible. Place the plate in a vertical position, and evenly spray with Spray solution 1 until the adsorbent layer is uniformly soaked down to the glass (do not overspray). Place the plate in a horizontal position, and dry as completely as possible with the aid of a current of warm dry air (no odor of hydrochloric acid is perceptible). Place the plate in a vertical position, and evenly spray with Spray solution 2 until the plate is uniformly wet (do not overspray). The major methyldopa spot is black on a pale pink or orange background at an RF value of about 0.50, and the 3-O-methylmethyldopa spot is dark on a similar background at an RF value of about 0.65. The area and intensity of any 3-O-methylmethyldopa spot from the Test solution are not greater than those from the Standard solution (0.5%).
Assay—
Dissolve about 200 mg of Methyldopa, accurately weighed, in 25 mL of glacial acetic acid, with the aid of heat. Cool to room temperature, and add 0.1 mL of crystal violet TS and 50 mL of acetonitrile. Titrate with 0.1 N perchloric acid VS to a blue endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 21.12 mg of C10H13NO4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Sujatha Ramakrishna, Ph.D.
Scientist 1-301-816-8349 |
(MDCV05) Monograph Development-Cardiovascular |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2940
Pharmacopeial Forum: Volume No. 28(3) Page 769
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.