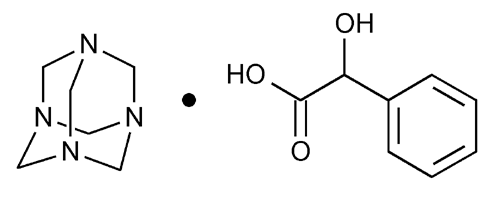
Methenamine Mandelate
Benzeneacetic acid,
Hexamethylenetetramine mono-(±)-mandelate
» Methenamine Mandelate contains not less than 95.5 percent and not more than 102.0 percent of C6H12N4·C8H8O3, and contains not less than 50.0 percent and not more than 53.0 percent of mandelic acid (C8H8O3), calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification, Infrared Absorption  197K
197K .
.
Loss on drying  731
731 —
Dry it over silica gel for 18 hours: it loses not more than 1.5% of its weight.
—
Dry it over silica gel for 18 hours: it loses not more than 1.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Chloride  221
221 —
Dissolve 1.0 g in 10 mL of water, and add gradually 500 mg of anhydrous sodium carbonate. Evaporate to dryness, and ignite the residue at a dull-red heat. Add 20 mL of 2 N nitric acid, stir gently, and filter: the filtrate shows no more chloride than corresponds to 0.15 mL of 0.020 N hydrochloric acid (0.01%).
—
Dissolve 1.0 g in 10 mL of water, and add gradually 500 mg of anhydrous sodium carbonate. Evaporate to dryness, and ignite the residue at a dull-red heat. Add 20 mL of 2 N nitric acid, stir gently, and filter: the filtrate shows no more chloride than corresponds to 0.15 mL of 0.020 N hydrochloric acid (0.01%).
Sulfate—
Dissolve 0.20 g in 10 mL of water, and add 5 drops of 3 N hydrochloric acid and 5 drops of barium chloride TS: no turbidity appears within 1 minute.
Heavy metals  231
231 —
Dissolve 1.3 g in 10 mL of water, add 2 mL of 3 N hydrochloric acid, and dilute with water to 25 mL: the limit is 0.0015%.
—
Dissolve 1.3 g in 10 mL of water, add 2 mL of 3 N hydrochloric acid, and dilute with water to 25 mL: the limit is 0.0015%.
Mandelic acid content—
Transfer about 90 mg, accurately weighed, to a 250-mL conical flask containing 50 mL of water. When solution is complete, titrate the magnetically stirred solution with 0.05 N ceric ammonium nitrate VS, determining the endpoint potentiometrically. Each mL of 0.05 N ceric ammonium nitrate is equivalent to 3.804 mg of C8H8O3.
Assay—
0.05 N Silver nitrate in dehydrated alcohol—
Dissolve, by stirring, about 8.5 g of silver nitrate in 1000 mL of dehydrated alcohol. Transfer about 100 mg, accurately weighed, of sodium chloride, previously dried at 110 for 2 hours, to a 100-mL beaker, and dissolve in 50 mL of water. Titrate with the silver nitrate solution to the potentiometric endpoint, using a silver billet indicator electrode and a silver-silver chloride double-junction reference electrode containing a potassium nitrate salt bridge. Calculate the normality of the titrant.
for 2 hours, to a 100-mL beaker, and dissolve in 50 mL of water. Titrate with the silver nitrate solution to the potentiometric endpoint, using a silver billet indicator electrode and a silver-silver chloride double-junction reference electrode containing a potassium nitrate salt bridge. Calculate the normality of the titrant.
Procedure—
Transfer about 60 mg of Methenamine Mandelate, accurately weighed, to a 250-mL conical flask. Add 15 mL of dehydrated alcohol, stir to dissolve, and add 40 mL of chloroform. Titrate with 0.05 N Silver nitrate in dehydrated alcohol, determining the endpoint potentiometrically, using a silver billet indicator electrode and a silver-silver chloride double-junction reference electrode containing a potassium nitrate salt bridge. Each mL of 0.05 N silver nitrate is equivalent to 7.308 mg of C6H12N4·C8H8O3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientist 1-301-816-8394 |
(MDAA05) Monograph Development-Antivirals and Antimicrobials |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2922
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.