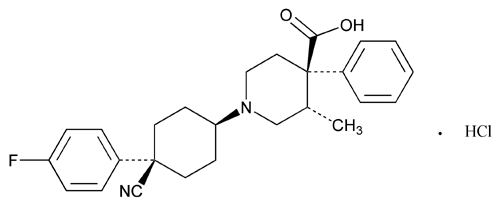
Levocabastine Hydrochloride
4-Piperidinecarboxylic acid, 1-[4-cyano-4-(4-fluorophenyl)cyclohexyl]-3-methyl-4-phenyl-, monohydrochloride, (–)-[1(cis),3
(–)-trans-1-[cis-4-Cyano-4-(p-fluorophenyl)cyclohexyl]-3-methyl-4-phenylisonipecotic acid monohydrochloride
» Levocabastine Hydrochloride contains not less than 98.5 percent and not more than 101.5 percent of C26H29FN2O2·HCl, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers. Protect from light.
USP Reference standards  11
11 —
—
USP Levocabastine Hydrochloride RS .
.
USP Levocabastine Related Compound A RS.
USP Levocabastine Hydrochloride RS
 .
.
USP Levocabastine Related Compound A RS.
Identification—
A:
Infrared Absorption  197K
197K .
.
B:
It meets the requirements of the tests for Chloride  191
191 .
.
C:
It meets the requirement for Specific rotation  781S
781S .
.
Specific rotation  781S
781S :
between –102
:
between –102 and –106
and –106 at 20
at 20 .
.
Test solution:
10 mg per mL, in methanol.
Loss on drying  731
731 —
Dry about 1.000 g of sample at 105
—
Dry about 1.000 g of sample at 105 to constant weight: it loses not more than 0.5% of its weight.
to constant weight: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%, based on a sample weight of about 1.000 g.
:
not more than 0.1%, based on a sample weight of about 1.000 g.
Related compounds—
[note—Prepare solutions immediately before use.]
Diluent—
Prepare an aqueous solution containing about 2 mg per mL of sodium hydroxide.
pH 9.0 Buffer—
Dissolve about 1.39 g of boric acid in water, and adjust with 1 N sodium hydroxide to a pH of 9.0. Dilute with water to 100 mL.
Run buffer—
Dissolve about 1.08 g of sodium dodecyl sulfate and about 650 mg of hydroxypropyl-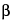 -cyclodextrin in 5 mL of isopropyl alcohol, then dilute with pH 9.0 Buffer to 50 mL.
-cyclodextrin in 5 mL of isopropyl alcohol, then dilute with pH 9.0 Buffer to 50 mL.
System suitability solution—
Dissolve suitable quantities of USP Levocabastine Hydrochloride RS and USP Levocabastine Related Compound A RS in Diluent to obtain a solution containing about 0.0125 mg per mL of each compound.
Test solution—
Transfer about 25 mg of Levocabastine Hydrochloride, accurately weighed, to a 10-mL volumetric flask, dissolve in and dilute with Diluent to volume, and mix.
Standard solution—
Dilute 5.0 mL of the Test solution with Diluent to 100 mL. Dilute 1.0 mL of this solution with Diluent to 10 mL to obtain a solution containing about 0.0125 mg of levocabastine hydrochloride per mL.
System setup (see Capillary Electrophoresis  727
727 )—
The capillary electrophoresis system is equipped with a 214-nm detector and a 75-µm × 50-cm uncoated fused-silica capillary column. The temperature is maintained at 50
)—
The capillary electrophoresis system is equipped with a 214-nm detector and a 75-µm × 50-cm uncoated fused-silica capillary column. The temperature is maintained at 50 . The current is programmed as follows.
. The current is programmed as follows.
Equilibrate the capillary column with the Diluent for 2 minutes, then equilibrate with the Run buffer for at least 5 minutes. Inject the System suitability solution, and record the peak responses as directed for Procedure: the relative migration times are approximately 1.07 for levocabastine related compound A and 1.0 for levocabastine; the resolution, R, between levocabastine and levocabastine related compound A is not less than 4. If necessary, adjust the current gradient to achieve the required resolution.
| Time (minutes) | Current (µA) |
| 0–0.17 | 0®75 |
| 0.17–15 | 75®130 |
| 15–40 | 130 |
| 40–60 | 130®200 |
Procedure—
Separately inject equal volumes (pressure of 3450 Pa for 5 seconds) of the Diluent (as the blank), the Standard solution, and the Test solution, and record the peak responses: the area for any peak in the Test solution, other than the major peak, is not greater than the major peak area obtained from the Standard solution (0.5%); and the sum of all peak areas in the Test solution, except for the major peak, is not greater than twice the major peak area obtained from the Standard solution (1.0%). Disregard any peak originating from the Diluent. Disregard any peak with an area of less than 0.1 times the major peak area obtained from the Standard solution (0.05%).
Assay—
Dissolve about 175 mg of Levocabastine Hydrochloride, accurately weighed, in 50 mL of alcohol, and add 5.0 mL of 0.01 N hydrochloric acid. Titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically. The volume of titrant required to titrate Levocabastine Hydrochloride is the difference between the first and third endpoints. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of 0.1 N sodium hydroxide VS is equivalent to 22.85 mg of C26H29FN2O2·HCl.
). Each mL of 0.1 N sodium hydroxide VS is equivalent to 22.85 mg of C26H29FN2O2·HCl.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2768
Pharmacopeial Forum: Volume No. 31(6) Page 1647