Iothalamate Sodium Injection
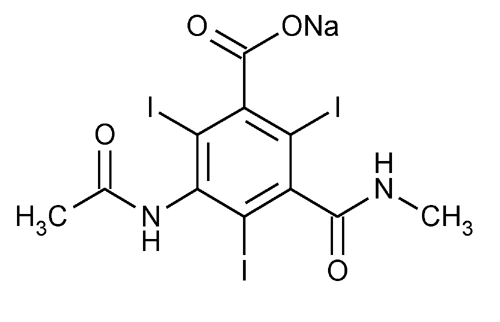
Benzoic acid, 3-(acetylamino)-2,4,6-triiodo-5-[(methylamino)carbonyl]-, monosodium salt.
Monosodium 5-acetamido-2,4,6-triiodo-N-methylisophthalamate
» Iothalamate Sodium Injection is a sterile solution of Iothalamic Acid in Water for Injection prepared with the aid of Sodium Hydroxide. It contains not less than 95.0 percent and not more than 105.0 percent of the labeled amount of iothalamate sodium (C11H8I3N2NaO4). It may contain small amounts of suitable buffers and of Edetate Calcium Disodium or Edetate Disodium as a stabilizer. Iothalamate Sodium Injection intended for intravascular use contains no antimicrobial agents.
Packaging and storage—
Preserve in single-dose containers, preferably of Type I glass, protected from light.
Labeling—
Label containers of the Injection intended for intravascular injection to direct the user to discard any unused portion remaining in the container. Label containers of the Injection intended for other than intravascular injection to show that the contents are not intended for intravascular injection.
USP Reference standards  11
11 —
—
USP 5-Amino-2,4,6-triiodo-N-methylisophthalamic Acid RS.
USP Endotoxin RS.
USP Iothalamic Acid RS.
USP 5-Amino-2,4,6-triiodo-N-methylisophthalamic Acid RS.
USP Endotoxin RS.
USP Iothalamic Acid RS.
Identification—
A:
Dilute 3 mL of Injection with water to 100 mL, add an excess of 3 N hydrochloric acid, and filter. Wash the precipitated iothalamic acid with four 10-mL portions of water, and dry at 105 for 4 hours: the dried iothalamic acid responds to Identification tests A and B under Iothalamate Meglumine Injection.
for 4 hours: the dried iothalamic acid responds to Identification tests A and B under Iothalamate Meglumine Injection.
B:
It responds to the flame test for Sodium  191
191 .
.
Bacterial endotoxins  85
85 —
It contains not more than 3.35 USP Endotoxin Units per mL.
—
It contains not more than 3.35 USP Endotoxin Units per mL.
pH  791
791 :
between 6.5 and 7.7.
:
between 6.5 and 7.7.
Free aromatic amine—
Dilute a suitable volume of Injection with water to yield a solution containing 100 mg of iothalamate sodium per mL. Proceed as directed in the test for Free aromatic amine under Iothalamic Acid, beginning with “Pipet 5 mL of this solution into a 50-mL volumetric flask.”
Iodine and iodide—
Dilute a volume of Injection, equivalent to about 2 g of iothalamate sodium, with 20 mL of water in a 50-mL beaker, add 5 mL of 2 N sulfuric acid, stir, and filter into a glass-stoppered, 50-mL cylinder. Proceed as directed for Procedure in the test for Iodine and iodide under Iothalamic Acid, beginning with “To the filtrate add 5 mL of toluene.”
Heavy metals  231
231 —
In a 50-mL color-comparison tube, mix a volume of Injection, equivalent to 1.0 g of iothalamate sodium, with 5 mL of 1 N sodium hydroxide, dilute with water to 40 mL, and mix. Using this as the Test preparation, proceed as directed in the test for Heavy metals under Diatrizoate Meglumine: the limit is 0.002%.
—
In a 50-mL color-comparison tube, mix a volume of Injection, equivalent to 1.0 g of iothalamate sodium, with 5 mL of 1 N sodium hydroxide, dilute with water to 40 mL, and mix. Using this as the Test preparation, proceed as directed in the test for Heavy metals under Diatrizoate Meglumine: the limit is 0.002%.
Other requirements—
It meets the requirements under Injections  1
1 .
.
Assay—
Proceed with Injection as directed in the Assay under Iothalamate Meglumine Injection. Each mL of 30.05 N silver nitrate is equivalent to 10.60 mg of C11H8I3N2NaO4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(RMI05) Radiopharmaceuticals and Medical Imaging Agents 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2676