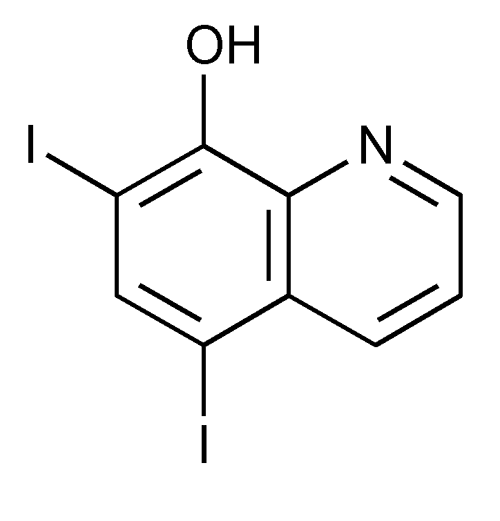
Iodoquinol
» Iodoquinol contains not less than 96.0 percent and not more than 100.5 percent of C9H5I2NO, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Prepare a 1 in 200 solution in carbon disulfide, warming slightly, if necessary, to effect complete solution: the IR absorption spectrum of this solution, in a 3-mm sodium chloride cell, carbon disulfide being used as the blank, in the region from 7 µm to 11 µm exhibits absorption maxima and minima only at the same wavelengths as that of a similar solution of USP Iodoquinol RS, concomitantly measured.
B:
Warm a small quantity of it with 1 mL of sulfuric acid: violet vapors of iodine are evolved.
Loss on drying  731
731 —
Dry it over silica gel for 4 hours: it loses not more than 0.5% of its weight.
—
Dry it over silica gel for 4 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.5%.
:
not more than 0.5%.
Free iodine and iodide—
Shake 1.0 g with 20 mL of water for 30 seconds, allow to stand for 5 minutes, and filter. To 10 mL of the filtrate add 1 mL of 2 N sulfuric acid, then add 2 mL of chloroform, and shake: no violet color appears in the chloroform (free iodine). To the mixture add 5 mL of 2 N sulfuric acid and 1 mL of potassium dichromate TS, and shake for 15 seconds: the color in the chloroform layer is not deeper than that produced in a control test made in the following manner. Dilute 2 mL of potassium iodide solution (1 in 6000) with water to 10 mL, add 6 mL of 2 N sulfuric acid, 1 mL of potassium dichromate TS, and 2 mL of chloroform, and shake for 15 seconds (0.05% of iodide).
Assay—
Using about 14 mg of Iodoquinol, accurately weighed, proceed as directed under Oxygen Flask Combustion  471
471 , using a mixture of 10 mL of sodium hydroxide solution (1 in 100) and 1 mL of freshly prepared sodium bisulfite solution (1 in 100) as the absorbing liquid. When the combustion is complete, place a few mL of water around the stopper of the flask, loosen the stopper, then rinse the stopper, the specimen holder, and the sides of the flask with about 20 mL of water, added in small portions. Add 1 mL of an oxidizing solution prepared by adding 5 mL of bromine to 100 mL of a 1 in 10 solution of sodium acetate in glacial acetic acid. Insert the stopper in the flask, and shake vigorously for 1 minute. Add 0.5 mL of formic acid, replace the stopper, and shake vigorously for 1 minute. Remove the stopper, and rinse the stopper, the specimen holder, and the sides of the flask with several small portions of water. Bubble nitrogen through the flask to remove the oxygen and excess bromine, add 500 mg of potassium iodide, swirl to dissolve, add 3 mL of 2 N sulfuric acid, swirl to mix, and allow to stand for 2 minutes. Titrate with 0.02 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Each mL of 0.02 N sodium thiosulfate is equivalent to 0.6616 mg of C9H5I2NO.
, using a mixture of 10 mL of sodium hydroxide solution (1 in 100) and 1 mL of freshly prepared sodium bisulfite solution (1 in 100) as the absorbing liquid. When the combustion is complete, place a few mL of water around the stopper of the flask, loosen the stopper, then rinse the stopper, the specimen holder, and the sides of the flask with about 20 mL of water, added in small portions. Add 1 mL of an oxidizing solution prepared by adding 5 mL of bromine to 100 mL of a 1 in 10 solution of sodium acetate in glacial acetic acid. Insert the stopper in the flask, and shake vigorously for 1 minute. Add 0.5 mL of formic acid, replace the stopper, and shake vigorously for 1 minute. Remove the stopper, and rinse the stopper, the specimen holder, and the sides of the flask with several small portions of water. Bubble nitrogen through the flask to remove the oxygen and excess bromine, add 500 mg of potassium iodide, swirl to dissolve, add 3 mL of 2 N sulfuric acid, swirl to mix, and allow to stand for 2 minutes. Titrate with 0.02 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Each mL of 0.02 N sodium thiosulfate is equivalent to 0.6616 mg of C9H5I2NO.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientist 1-301-816-8394 |
(MDAA05) Monograph Development-Antivirals and Antimicrobials |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2665