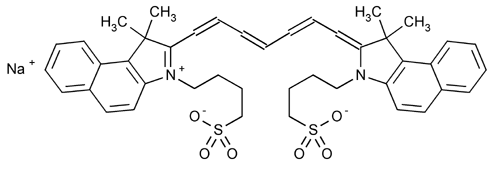
Indocyanine Green
1H-Benz[e]indolium, 2-[7-[1,3-dihydro-1,1-dimethyl-3-(4-sulfobutyl)-2H-benz[e]indol-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-, hydroxide, inner salt, sodium salt.
2-[7-[1,1-Dimethyl-3-(4-sulfobutyl)benz[e]indolin-2-ylidene]-1,3,5-heptatrienyl]-1,1-dimethyl-3-(4-sulfobutyl)-1Hbenz[e]indolium hydroxide, inner salt, sodium salt
» Indocyanine Green contains not less than 89.0 percent and not more than 100.0 percent of C43H47N2NaO6S2, calculated on the dried basis. It contains not more than 5.0 percent of sodium iodide, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
A:
Incinerate a portion of it: the residue responds to the tests for Sodium  191
191 and for Sulfate
and for Sulfate  191
191 .
.
B:
To a solution (1 in 20,000) add 10 drops of 1 N sodium hydroxide, and heat to about 60 . Add 10 drops of hydrogen peroxide TS, and mix: a red color develops within about 4 minutes and, on standing, fades to a pale orange.
. Add 10 drops of hydrogen peroxide TS, and mix: a red color develops within about 4 minutes and, on standing, fades to a pale orange.
Loss on drying  731
731 —
Dry it in vacuum at 50
—
Dry it in vacuum at 50 for 3 hours: it loses not more than 6.0% of its weight.
for 3 hours: it loses not more than 6.0% of its weight.
Arsenic, Method II  211
211 :
8 ppm.
:
8 ppm.
Lead—
A 5-mL portion of the solution prepared for the test for Arsenic  211
211 contains not more than 2 µg of lead (corresponding to not more than 0.001%) when tested by the limit test for Lead
contains not more than 2 µg of lead (corresponding to not more than 0.001%) when tested by the limit test for Lead  251
251 , 3 mL of Ammonium Citrate Solution, 1 mL of Potassium Cyanide Solution, and 0.5 mL of Hydroxylamine Hydrochloride Solution being used.
, 3 mL of Ammonium Citrate Solution, 1 mL of Potassium Cyanide Solution, and 0.5 mL of Hydroxylamine Hydrochloride Solution being used.
Sodium iodide—
Dissolve about 200 mg, accurately weighed, in 100 mL of water, add 1 mL of nitric acid, mix, and titrate with 0.01 N silver nitrate VS, determining the endpoint potentiometrically, using silver and glass electrodes. Each mL of 0.01 N silver nitrate is equivalent to 1.499 mg of sodium iodide. Not more than 5.0% of sodium iodide, calculated on the dried basis, is found.
Other requirements—
Where the label states that Indocyanine Green is sterile, it meets the requirements for Sterility Tests  71
71 and for Bacterial endotoxins under Indocyanine Green for Injection. Where the label states that Indocyanine Green must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Indocyanine Green for Injection.
and for Bacterial endotoxins under Indocyanine Green for Injection. Where the label states that Indocyanine Green must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Indocyanine Green for Injection.
Assay—
Dissolve a quantity of Indocyanine Green, equivalent to about 100 mg of dried indocyanine green and accurately weighed, in methanol, and dilute quantitatively and stepwise with methanol to obtain a solution containing about 2 µg per mL. Dissolve a quantity of USP Indocyanine Green RS, accurately weighed, in methanol, and dilute quantitatively and stepwise with methanol to obtain a Standard solution having a known concentration of about 2 µg per mL. Concomitantly determine the absorbances of both solutions in 1-cm cells at the wavelength of maximum absorbance at about 785 nm, with a suitable spectrophotometer, using methanol as the blank. Calculate the quantity, in mg, of C43H47N2NaO6S2 in the Indocyanine Green taken by the formula:
50C(AU / AS)
in which C is the concentration, in µg per mL, of USP Indocyanine Green RS in the Standard solution; and AU and AS are the absorbances of the solution of Indocyanine Green and the Standard solution, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(RMI05) Radiopharmaceuticals and Medical Imaging Agents 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2631
Pharmacopeial Forum: Volume No. 29(6) Page 1905