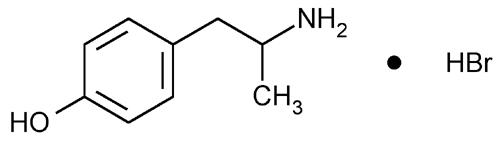
Hydroxyamphetamine Hydrobromide
Phenol, 4-(2-aminopropyl)-, hydrobromide.
(±)-p-(2-Aminopropyl)phenol hydrobromide
» Hydroxyamphetamine Hydrobromide contains not less than 98.0 percent and not more than 101.5 percent of C9H13NO·HBr, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed, light-resistant containers.
Identification—
B:
Dissolve about 500 mg of ammonium molybdate in 10 mL of sulfuric acid, and add to this solution about 2 mg of Hydroxyamphetamine Hydrobromide: an intense blue color is produced (distinction from similar amino compounds such as amphetamine and methamphetamine, which, lacking a phenolic hydroxyl, do not undergo this reaction).
C:
Dissolve about 200 mg in 2 mL of water, and add a solution of 500 mg of potassium carbonate in 2 mL of water. Extract with two 10-mL portions of ether, allow the clear ether solution to evaporate to dryness, and dry at about 80 : the hydroxyamphetamine so obtained melts between 124
: the hydroxyamphetamine so obtained melts between 124 and 127
and 127 (see Class I under Melting Range or Temperature
(see Class I under Melting Range or Temperature  741
741 ).
).
D:
To a solution of about 10 mg of it in 10 mL of water add 1 mL of 2 N nitric acid, then add silver nitrate TS: a pale yellow precipitate is formed, and it is slightly soluble in 6 N ammonium hydroxide.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 2 hours: it loses not more than 0.5% of its weight.
for 2 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Bromide content—
Accurately weigh about 400 mg, and dissolve in 50 mL of water. Add 50 mL of methanol and 10 mL of glacial acetic acid, then add eosin Y TS, and titrate with 0.1 N silver nitrate VS. Each mL of 0.1 N silver nitrate is equivalent to 7.990 mg of Br: the content of Br, calculated on the dried basis, is between 33.6% and 35.2%.
Ordinary impurities  466
466 —
—
Test solution:
methanol.
Standard solution:
methanol.
Eluant:
a mixture of toluene, methanol, and ammonium hydroxide (10:4:0.25).
Visualization:
1.
Assay—
Dissolve about 400 mg of Hydroxyamphetamine Hydrobromide, accurately weighed, in a mixture of 10 mL of glacial acetic acid and 10 mL of mercuric acetate TS, warming slightly, if necessary, to effect solution. Add crystal violet TS, and titrate with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 23.21 mg of C9H13NO·HBr.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Feiwen Mao, M.S.
Scientist 1-301-816-8320 |
(MDOOD05) Monograph Development-Ophthalmics Oncologics and Dermatologicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2591