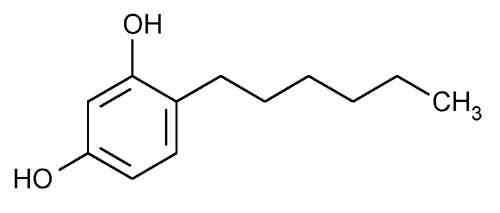
Hexylresorcinol
» Hexylresorcinol, dried over silica gel for 4 hours, contains not less than 98.0 percent and not more than 100.5 percent of C12H18O2.
Caution—Hexylresorcinol is irritating to the oral mucosa and respiratory tract and to the skin, and its solution in alcohol has vesicant properties.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
A:
To 1 mL of a saturated solution of it add 1 mL of nitric acid: a light red color appears.
B:
To 1 mL of a saturated solution of it add 1 mL of bromine TS: a yellow, flocculent precipitate is formed. Add 2 mL of 6 N ammonium hydroxide: the precipitate dissolves, producing a yellow solution.
Acidity—
Dissolve 250 mg in 500 mL of water, add methyl red TS, and titrate with 0.020 N sodium hydroxide: no more than 1.0 mL is required for neutralization.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Mercury—
[note—Select all reagents for this test to have as low a content of mercury as practicable, and store all reagent solutions in containers of borosilicate glass. Glassware used in this test shall be specially cleaned by being soaked in warm 8 N nitric acid for 30 minutes and rinsed with water. Keep flasks for this determination separate from other flasks, and use only for mercury determinations.]
Standard preparation—
Transfer 34.0 mg of mercuric chloride to a 250-mL volumetric flask. Add 1 drop of hydrochloric acid, add water to dissolve, and dilute with water to volume. Transfer 1.0 mL of this solution to a 100-mL volumetric flask, add 1 drop of hydrochloric acid, and dilute with water to volume. Transfer 1.0 mL of this solution to a 500-mL volumetric flask, add 1 drop of hydrochloric acid, and dilute with water to volume.
Test preparation—
Transfer 134 mg to a 250-mL beaker, and cautiously add 10 mL of 11 N nitric acid and 10 mL of 18 N sulfuric acid. Digest, with the aid of heat, in a well-ventilated hood until the evolution of brown fumes ceases. Cautiously add an additional 10 mL of 11 N nitric acid, and continue heating until no more fumes are evolved. Cool, transfer to a 200-mL volumetric flask, and dilute with water to volume.
Procedure—
Transfer 100 mL of Standard preparation to a 300-mL mercury analysis reaction vessel, add 2 drops of potassium permanganate solution (1 in 20), and mix (the solution should be purple; add additional permanganate solution dropwise, if necessary). Add 5 mL of 11 N nitric acid, stir, and allow to stand for not less than 15 seconds. Add 5 mL of 18 N sulfuric acid, stir, and allow to stand for not less than 45 seconds. Add 5 mL of hydroxylamine hydrochloride solution (3 in 200), stir, and allow to stand until the solution turns light yellow or colorless. Add 5 mL of stannous chloride solution (1 in 10) [note—Disregard the presence of insoluble matter in this solution; mix prior to use], immediately insert the aerator connected to the air pump, and determine the maximum absorbance of the treated Standard preparation at the mercury resonance line of 253.65 nm, with a suitable atomic absorption spectrophotometer equipped with a mercury hallow-cathode lamp and an absorption cell that permits the flameless detection of mercury. Connect in a closed system with a circulating air pump, a calcium chloride drying tube, and an aerator inserted in a 300-mL reaction vessel so that air passed through the treated preparation contained in the reaction vessel evaporates any metallic mercury present. In a similar manner, treat 100 mL of the Test preparation and 100 mL of water (reagent blank), and determine the maximum absorbances at the same wavelength [note—Check the zero setting of the instrument frequently]. The absorbance of the solution from the Test preparation does not exceed that of the solution from the Standard preparation (3 ppm).
Resorcinol and other phenols—
Shake about 1 g with 50 mL of water for a few minutes, filter, and to the filtrate add 3 drops of ferric chloride TS: no red or blue color is produced.
Assay—
Dissolve 70 mg to 100 mg of Hexylresorcinol, previously dried over silica gel for 4 hours and accurately weighed, in 10 mL of methanol in a 250-mL iodine flask. Add 30.0 mL of 0.1 N bromine VS, then add quickly 5 mL of hydrochloric acid, and insert the stopper in the flask immediately. Cool the flask under running water to room temperature, shake vigorously for 5 minutes, then set aside for 5 minutes. Add 6 mL of potassium iodide TS around the stopper, cautiously loosen the stopper, again insert the stopper tightly, and swirl gently. Add 1 mL of chloroform, and titrate the liberated iodine with 0.1 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Perform a blank determination (see Residual Titrations under Titrimetry  541
541 ). Each mL of 0.1 N bromine is equivalent to 4.857 mg of C12H18O2.
). Each mL of 0.1 N bromine is equivalent to 4.857 mg of C12H18O2.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientist 1-301-816-8394 |
(MDAA05) Monograph Development-Antivirals and Antimicrobials |
USP32–NF27 Page 2556