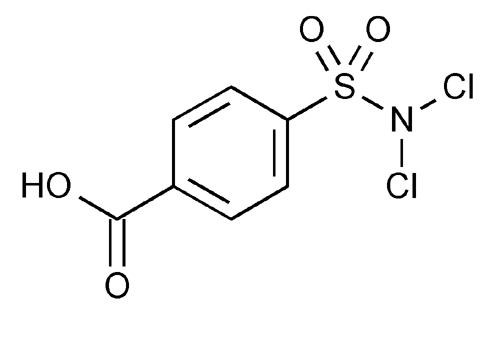
Halazone
Benzoic acid, 4-[(dichloroamino)sulfonyl]-.
p-(Dichlorosulfamoyl)benzoic acid
» Halazone contains not less than 91.5 percent and not more than 100.5 percent of C7H5Cl2NO4S, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
Add about 100 mg to 5 mL of sodium bromide solution (1 in 10): bromine is liberated from the mixture.
Loss on drying  731
731 —
Dry it over phosphorus pentoxide for 4 hours: it loses not more than 0.5% of its weight.
—
Dry it over phosphorus pentoxide for 4 hours: it loses not more than 0.5% of its weight.
Readily carbonizable substances  271
271 —
Dissolve 100 mg in 0.5 mL of sulfuric acid TS: no blackening occurs, although some effervescence may take place.
—
Dissolve 100 mg in 0.5 mL of sulfuric acid TS: no blackening occurs, although some effervescence may take place.
Assay—
Add about 150 mg of Halazone, accurately weighed, to 10 mL of 2.5 N sodium hydroxide in a 250-mL iodine flask, stir well to dissolve, add 75 mL of water, then promptly add 15 mL of potassium iodide solution (1 in 10), and mix. Acidify with 10 mL of 6 N acetic acid, and immediately titrate the liberated iodine with 0.1 N sodium thiosulfate VS, adding 3 mL of starch TS as the endpoint is approached. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N sodium thiosulfate is equivalent to 6.752 mg of C7H5Cl2NO4S.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(GTMDB05) General Toxicology and Medical Device Biocompatibility |
USP32–NF27 Page 2545
Pharmacopeial Forum: Volume No. 34(5) Page 1163