Gemcitabine for Injection
» Gemcitabine for Injection contains an amount of Gemcitabine Hydrochloride equivalent to not less than 95 percent and not more than 105 percent of the labeled amount of gemcitabine (C9H11F2N3O4).
Caution—Gemcitabine Hydrochloride is a potent cytotoxic agent. Great care should be taken to prevent inhaling particles and exposing the skin to it.
Packaging and storage—
Preserve in Containers for Sterile Solids, as described under Injections  1
1 . Store at controlled room temperature. Do not refrigerate after reconstitution.
. Store at controlled room temperature. Do not refrigerate after reconstitution.
Identification—
A:
Ultraviolet Absorption  197U
197U .
.
Solution:
16 µg per mL.
Medium:
0.14 M phosphate buffer with a pH of 2.5, prepared as follows. Add 13.8 g of monobasic sodium phosphate and 2.5 mL of phosphoric acid to 1000 mL of Purified Water.
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Clarity of solution—
Dissolve it in the solvent and at the concentration recommended in the labeling: not more than 10 NTU (see Spectrophotometry and Light-Scattering  851
851 ), determined by ratio turbidimetry within 15 minutes of reconstitution, corrected for a diluent blank.
), determined by ratio turbidimetry within 15 minutes of reconstitution, corrected for a diluent blank.
Bacterial endotoxins  85
85 —
It contains not more than 0.05 USP Endotoxin Unit per mg of gemcitabine.
—
It contains not more than 0.05 USP Endotoxin Unit per mg of gemcitabine.
Sterility  71
71 —
It meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined.
—
It meets the requirements when tested as directed for Membrane Filtration under Test for Sterility of the Product to be Examined.
Uniformity of dosage units  905
905 :
meets the requirements for Weight Variation.
:
meets the requirements for Weight Variation.
pH  791
791 :
between 2.7 and 3.3, in a solution containing 40 mg in each mL of 0.9% sodium chloride solution.
:
between 2.7 and 3.3, in a solution containing 40 mg in each mL of 0.9% sodium chloride solution.
Particulate matter  788
788 :
meets the requirements for small-volume injections.
:
meets the requirements for small-volume injections.
Chromatographic purity—
Mobile phase, System suitability solution, Standard solution, and Chromatographic system—
Proceed as directed in the test for Chromatographic purity for Gemcitabine Hydrochloride.
Test solution—
Reconstitute the vial with an appropriate amount of water to achieve a solution of 2 mg per mL, based on the labeled content of gemcitabine.
Procedure—
Proceed as directed in Chromatographic purity for Gemcitabine Hydrochloride. Calculate the amount of cytosine, expressed as a percentage of gemcitabine hydrochloride, by the formula:
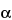 -anomer or any other individual impurity in the Test solution; and rS is the peak response for gemcitabine in the Standard solution. Not more than 0.1% of gemcitabine
-anomer or any other individual impurity in the Test solution; and rS is the peak response for gemcitabine in the Standard solution. Not more than 0.1% of gemcitabine 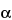 -anomer is found; not more than 0.2% each of any other impurity is found; and the sum of all impurities is not more than 0.3%. Exclude from the sum of all impurities any peaks that are below the limit of quantitation (0.02%).
-anomer is found; not more than 0.2% each of any other impurity is found; and the sum of all impurities is not more than 0.3%. Exclude from the sum of all impurities any peaks that are below the limit of quantitation (0.02%).
0.1(263.20/299.66)(CCV/L)(rt / rS)
in which 263.20 and 299.66 are the molecular weights of gemcitabine and gemcitabine hydrochloride, respectively; CC is the concentration of USP Cytosine RS in the Standard solution, in µg per mL; V is the volume, in mL, of water used to reconstitute the vial; L is the labeled amount of gemcitabine in the vial, in mg; rt is the peak response for cytosine in the Test solution; and rS is the response for cytosine in the Standard solution: not more than 0.1% of cytosine is found. Similarly, calculate the amount of each impurity other than cytosine, expressed as a percentage of gemcitabine hydrochloride, by the formula:
0.1(263.20/299.66)(CSV/L)(ri / rS)
in which 263.20 and 299.66 are the molecular weights of gemcitabine and gemcitabine hydrochloride, respectively; CS is the concentration of USP Gemcitabine Hydrochloride RS in the Standard solution, in µg per mL; V is the volume, in mL, of water used to reconstitute the vial; L is the labeled amount of gemcitabine in the vial, in mg; ri is the response for gemcitabine
Assay—
Mobile phase, System suitability solution, Standard preparation, and Chromatographic system—
Proceed as directed in the Assay under Gemcitabine Hydrochloride.
Assay preparation—
Constitute a suitable number of vials of Gemcitabine for Injection with Purified Water to obtain a solution having a concentration of about 0.1 mg per mL, based on the labeled content of gemcitabine.
Procedure—
Proceed as directed in the Assay under Gemcitabine Hydrochloride. Calculate the amount, in mg, of gemcitabine (C9H11F2N3O4) in each vial of Gemcitabine for Injection taken by the formula:
(263.20/299.66)(CV/N)(rU / rS)
in which 263.20 and 299.66 are the molecular weights of gemcitabine and gemcitabine hydrochloride, respectively; V is the total volume, in mL, of the Assay preparation; N is the number of vials taken; and the other terms are as defined therein.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Feiwen Mao, M.S.
Scientist 1-301-816-8320 |
(MDOOD05) Monograph Development-Ophthalmics Oncologics and Dermatologicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2486
Pharmacopeial Forum: Volume No. 31(6) Page 1630
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.
