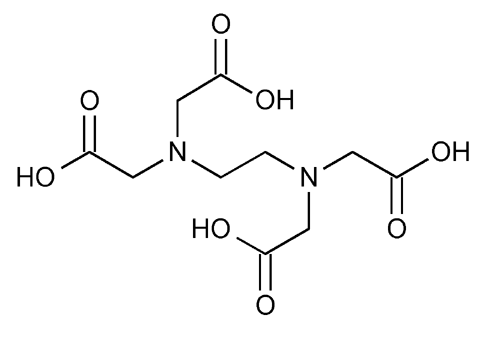
Edetic Acid
Glycine, N,N¢-1,2-ethanediylbis[N-(carboxymethyl)-.
(Ethylenedinitrilo)tetraacetic acid
» Edetic Acid contains not less than 98.0 percent and not more than 100.5 percent of C10H16N2O8.
Packaging and storage—
Preserve in well-closed containers.
Identification, Infrared Absorption  197K
197K .
.
Residue on ignition  281
281 :
not more than 0.2%.
:
not more than 0.2%.
Heavy metals, Method II  231
231 :
0.003%.
:
0.003%.
Iron—
Char 3.0 g thoroughly, and heat in an oven at 500 until most of the carbon is consumed. Cool, add 0.15 mL of nitric acid, and heat at 500
until most of the carbon is consumed. Cool, add 0.15 mL of nitric acid, and heat at 500 until all of the carbon is consumed. Dissolve the residue in 2 mL of a mixture of equal volumes of hydrochloric acid and water, digest in a covered dish on a steam bath for 10 minutes, remove the cover, and evaporate to dryness. Dissolve the residue in 1 mL of 1 N acetic acid and 20 mL of hot water, digest for 5 minutes on a steam bath, cool, and dilute with water to 30 mL. To 2.0 mL of this solution add 2 mL of hydrochloric acid, and dilute with water to 50 mL. Add about 50 mg of ammonium persulfate and 3 mL of ammonium thiocyanate solution (3 in 10), mix, and transfer to a color-comparison tube. Treat in the same manner 2.0 mL of a solution of ferric ammonium sulfate, prepared by dissolving 43.2 mg of ferric ammonium sulfate in 10 mL of 2 N sulfuric acid and adding water to make 1000 mL, each mL representing 5 µg of Fe. The color of the test solution is not deeper than that of the solution containing the standard iron solution (0.005%).
until all of the carbon is consumed. Dissolve the residue in 2 mL of a mixture of equal volumes of hydrochloric acid and water, digest in a covered dish on a steam bath for 10 minutes, remove the cover, and evaporate to dryness. Dissolve the residue in 1 mL of 1 N acetic acid and 20 mL of hot water, digest for 5 minutes on a steam bath, cool, and dilute with water to 30 mL. To 2.0 mL of this solution add 2 mL of hydrochloric acid, and dilute with water to 50 mL. Add about 50 mg of ammonium persulfate and 3 mL of ammonium thiocyanate solution (3 in 10), mix, and transfer to a color-comparison tube. Treat in the same manner 2.0 mL of a solution of ferric ammonium sulfate, prepared by dissolving 43.2 mg of ferric ammonium sulfate in 10 mL of 2 N sulfuric acid and adding water to make 1000 mL, each mL representing 5 µg of Fe. The color of the test solution is not deeper than that of the solution containing the standard iron solution (0.005%).
Limit of nitrilotriacetic acid—
Mobile phase
, Cupric nitrate solution, Stock standard solution, and Chromatographic system—Prepare as directed in the test for Limit of nitrilotriacetic acid under Edetate Disodium.
Resolution solution—
Using Edetic Acid instead of Edetate Disodium, prepare as directed for Resolution solution in the test for Limit of nitrilotriacetic acid under Edetate Disodium.
Standard preparation—
Transfer 1.0 g of Edetic Acid to a 100-mL volumetric flask, add 300 µL of Stock standard solution, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to achieve complete solution.
Test preparation—
Transfer 1.0 g of Edetic Acid to a 100-mL volumetric flask, dilute with Cupric nitrate solution to volume, and mix. Sonicate, if necessary, to achieve complete solution.
Procedure—
Proceed as directed for Procedure in the test for Limit of nitrilotriacetic acid under Edetate Disodium: the response of the nitrilotriacetic acid peak of the Test preparation does not exceed the difference between the nitrilotriacetic acid peak responses obtained from the Standard preparation and the Test preparation (0.3%).
Assay—
Assay preparation—
Transfer about 1.4 g of Edetic Acid, accurately weighed, to a 100-mL volumetric flask, dissolve in 11 mL of 1 N sodium hydroxide, dilute with water to volume, with cooling, if necessary, and mix.
Procedure—
Transfer to a 400-mL beaker about 200 mg of chelometric standard calcium carbonate, previously dried at 110 for 2 hours, cooled in a desiccator, and accurately weighed. Add 10 mL of water, swirl to form a slurry, and cover the beaker with a watch glass. Without removing the watch glass, add 2 mL of 3 N hydrochloric acid from a pipet, and swirl to dissolve. Wash down the sides of the beaker, the outer surface of the pipet, and the watch glass with water, and dilute with water to about 100 mL. While stirring with a magnetic stirrer, add about 30 mL of the Assay preparation from a 50-mL buret. Add 10 mL of 1 N sodium hydroxide and 300 mg of hydroxy naphthol blue, and continue the titration with the Assay preparation to a blue endpoint. Calculate the weight, in g, of C10H16N2O8 in the portion of Edetic Acid taken by the formula:
for 2 hours, cooled in a desiccator, and accurately weighed. Add 10 mL of water, swirl to form a slurry, and cover the beaker with a watch glass. Without removing the watch glass, add 2 mL of 3 N hydrochloric acid from a pipet, and swirl to dissolve. Wash down the sides of the beaker, the outer surface of the pipet, and the watch glass with water, and dilute with water to about 100 mL. While stirring with a magnetic stirrer, add about 30 mL of the Assay preparation from a 50-mL buret. Add 10 mL of 1 N sodium hydroxide and 300 mg of hydroxy naphthol blue, and continue the titration with the Assay preparation to a blue endpoint. Calculate the weight, in g, of C10H16N2O8 in the portion of Edetic Acid taken by the formula:
(292.24 / 100.09)(0.1W / V)
in which 292.24 and 100.09 are the molecular weights of edetic acid and calcium carbonate, respectively, W is the weight, in mg, of calcium carbonate, and V is the volume, in mL, of the Assay preparation consumed in the titration.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1230
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.