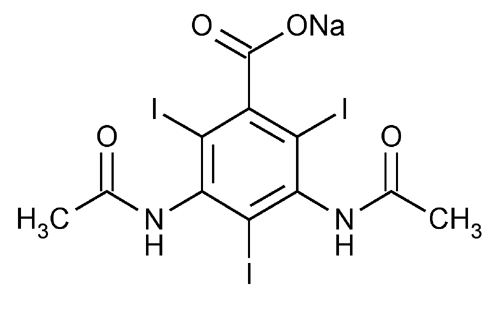
Diatrizoate Sodium
Benzoic acid, 3,5-bis(acetylamino)-2,4,6-triiodo-, monosodium salt.
Monosodium 3,5-diacetamido-2,4,6-triiodobenzoate
» Diatrizoate Sodium contains not less than 98.0 percent and not more than 102.0 percent of C11H8I3N2NaO4, calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers. Store at room temperature.
Identification—
A:
It responds to the Thin-layer Chromatographic Identification Test  201
201 , the test solution and the Standard solution being prepared at a concentration of 1 mg of USP Diatrizoic Acid RS per mL in a 0.8 in 1000 solution of sodium hydroxide in methanol, the solvent mixture being a mixture of chloroform, methanol, and ammonium hydroxide (20:10:2), and short-wavelength UV light being used to locate the spots.
, the test solution and the Standard solution being prepared at a concentration of 1 mg of USP Diatrizoic Acid RS per mL in a 0.8 in 1000 solution of sodium hydroxide in methanol, the solvent mixture being a mixture of chloroform, methanol, and ammonium hydroxide (20:10:2), and short-wavelength UV light being used to locate the spots.
B:
Heat about 500 mg in a suitable crucible: violet vapors are evolved.
C:
It responds to the flame test for Sodium  191
191 .
.
Water, Method I  921
921 :
not more than 10.0%.
:
not more than 10.0%.
Free aromatic amine—
Transfer 1.0 g to a 50-mL volumetric flask, and add 5 mL of water and 10 mL of 0.1 N sodium hydroxide. Proceed as directed in the test for Free aromatic amine under Diatrizoate Meglumine, beginning with “To a second 50-mL volumetric flask transfer 4 mL of water.”
Iodine and iodide—
Test preparation—
Transfer 2.0 g to a 50-mL centrifuge tube provided with a stopper, dilute with water to 24 mL, and shake to dissolve.
Procedure—
Proceed as directed for Procedure in the test for Iodine and iodide under Diatrizoate Meglumine.
Heavy metals  231
231 —
Dissolve 1.0 g of Diatrizoate Sodium in 20 mL of water and 5 mL of 1 N sodium hydroxide, transfer the solution to a 50-mL color-comparison tube, dilute with water to 40 mL, and mix. Using this as the Test preparation, proceed as directed for Heavy metals under Diatrizoate Meglumine: the limit is 0.002%.
—
Dissolve 1.0 g of Diatrizoate Sodium in 20 mL of water and 5 mL of 1 N sodium hydroxide, transfer the solution to a 50-mL color-comparison tube, dilute with water to 40 mL, and mix. Using this as the Test preparation, proceed as directed for Heavy metals under Diatrizoate Meglumine: the limit is 0.002%.
Assay—
Transfer about 300 mg of Diatrizoate Sodium, accurately weighed, to a glass-stoppered, 125-mL conical flask, add 30 mL of 1.25 N sodium hydroxide and 500 mg of powdered zinc, connect the flask to a reflux condenser, and reflux the mixture for 1 hour. Cool the flask to room temperature, rinse the condenser with 20 mL of water, disconnect the flask from the condenser, and filter the mixture. Rinse the flask and filter thoroughly, adding the rinsings to the filtrate. Add 5 mL of glacial acetic acid and 1 mL of tetrabromophenolphthalein ethyl ester TS, and titrate with 0.05 N silver nitrate VS until the yellow precipitate just turns green. Each mL of 0.05 N silver nitrate is equivalent to 10.60 mg of C11H8I3N2NaO4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(RMI05) Radiopharmaceuticals and Medical Imaging Agents 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2111
Pharmacopeial Forum: Volume No. 29(6) Page 1868