Deferoxamine Mesylate
Butanediamide, N¢-[5-[[4-[[5-(acetylhydroxyamino)pentyl]amino]-1,4-dioxobutyl]hydroxyamino]pentyl]-N-(5-aminopentyl)-N-hydroxy-, monomethanesulfonate.
N-[5-[3-[(5-Aminopentyl)hydroxycarbamoyl]propionamido]pentyl]-3-[[5-(N-hydroxyacetamido)pentyl]carbamoyl]propionohydroxamic acid monomethanesulfonate (salt)
» Deferoxamine Mesylate contains not less than 98.0 percent and not more than 102.0 percent of C25H48N6O8·CH4O3S, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
Dissolve 5 mg in 5 mL of water, add 2 mL of tribasic sodium phosphate solution (1 in 200), mix, then add 10 drops of 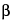 -naphthoquinone-4-sodium sulfonate solution (1 in 40): a blackish brown color is produced.
-naphthoquinone-4-sodium sulfonate solution (1 in 40): a blackish brown color is produced.
pH  791
791 :
between 4.0 and 6.0, in a solution (1 in 100).
:
between 4.0 and 6.0, in a solution (1 in 100).
Water, Method I  921
921 :
not more than 2.0%.
:
not more than 2.0%.
Residue on ignition  281
281 :
not more than 0.1%, 2.0 g being used.
:
not more than 0.1%, 2.0 g being used.
Chloride  221
221 —
A 1.2-g portion shows no more chloride than corresponds to 0.20 mL of 0.020 N hydrochloric acid (0.012%).
—
A 1.2-g portion shows no more chloride than corresponds to 0.20 mL of 0.020 N hydrochloric acid (0.012%).
Sulfate  221
221 —
A 0.5-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (0.04%).
—
A 0.5-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (0.04%).
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Other requirements—
Where the label states that Deferoxamine Mesylate is sterile, it meets the requirements for Sterility Tests  71
71 and Labeling under Injections
and Labeling under Injections  1
1 , and Bacterial endotoxins under Deferoxamine Mesylate for Injection. Where the label states that Deferoxamine Mesylate must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Deferoxamine Mesylate for Injection.
, and Bacterial endotoxins under Deferoxamine Mesylate for Injection. Where the label states that Deferoxamine Mesylate must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements for Bacterial endotoxins under Deferoxamine Mesylate for Injection.
Assay—
Ferric chloride solution—
Dissolve 6.7 g of ferric chloride in dilute hydrochloric acid (1 in 100) in a 100-mL volumetric flask. Add dilute hydrochloric acid (1 in 100) to volume, mix, and filter.
Standard preparation—
Dissolve a suitable quantity of USP Deferoxamine Mesylate RS, accurately weighed, in water to obtain a solution having a known concentration of about 1000 µg per mL.
Assay preparation—
Dissolve about 50 mg of Deferoxamine Mesylate, accurately weighed, in water to make 50.0 mL, and mix.
Procedure—
Pipet 2 mL each of the Standard preparation, the Assay preparation, and water to provide a blank, into separate 25-mL volumetric flasks. To each flask add 3 mL of Ferric chloride solution, dilute with water to volume, and mix. Concomitantly determine the absorbances of the solutions from the Standard preparation and the Assay preparation against the blank, in 1-cm cells, at the wavelength of maximum absorbance at about 485 nm, with a suitable spectrophotometer. Calculate the quantity, in mg, of C25H48N6O8·CH4O3S in the portion of Deferoxamine Mesylate taken by the formula:
0.05C(AU / AS)
in which C is the concentration, in µg per mL, of USP Deferoxamine Mesylate RS in the Standard preparation, and AU and AS are the absorbances of the solutions from the Assay preparation and Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2062
Pharmacopeial Forum: Volume No. 29(5) Page 1448
