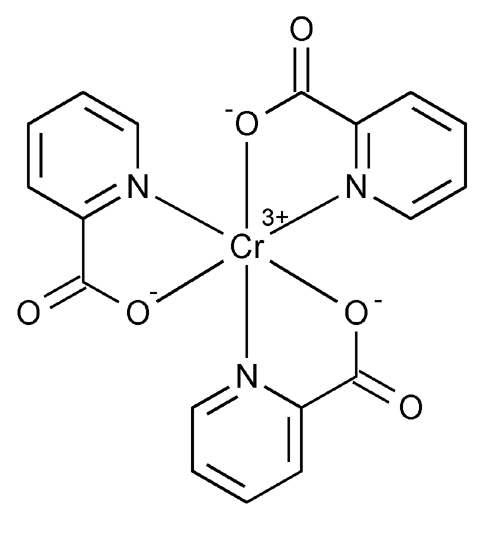
Chromium Picolinate
» Chromium Picolinate contains not less than 98.0 percent and not more than 102.0 percent of C18H12N3O6Cr, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
B:
To 5 mL of a solution (1 in 250) in a test tube add 1 mL of 5 N sodium hydroxide and 10 drops of 30% hydrogen peroxide, and heat gently for about 2 minutes: a yellow color develops.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 4 hours: it loses not more than 4.0% of its weight.
for 4 hours: it loses not more than 4.0% of its weight.
Chloride  221
221 —
Dissolve 30 mg of Chromium Picolinate in 30 to 40 mL of water, and heat to 70
—
Dissolve 30 mg of Chromium Picolinate in 30 to 40 mL of water, and heat to 70 . Cool overnight, and filter to remove the precipitate. Add 1 mL each of nitric acid and silver nitrate TS, and add sufficient water to make 50 mL. Mix, and allow to stand for 5 minutes, protected from direct sunlight: any turbidity formed is not greater than that produced in a similarly treated control solution containing 0.25 mL of 0.002 N hydrochloric acid (0.06%).
. Cool overnight, and filter to remove the precipitate. Add 1 mL each of nitric acid and silver nitrate TS, and add sufficient water to make 50 mL. Mix, and allow to stand for 5 minutes, protected from direct sunlight: any turbidity formed is not greater than that produced in a similarly treated control solution containing 0.25 mL of 0.002 N hydrochloric acid (0.06%).
Sulfate  221
221 —
Dissolve 100 mg of Chromium Picolinate in 30 to 40 mL of water, and heat to 90
—
Dissolve 100 mg of Chromium Picolinate in 30 to 40 mL of water, and heat to 90 . Cool overnight, and filter to remove the precipitate. Add 1 mL of 3 N hydrochloric acid, 3 mL of barium chloride TS, and sufficient water to make 50 mL. Mix, and allow to stand for 10 minutes: any turbidity formed is not greater than that produced in a similarly treated control solution containing 0.2 mL of 0.02 N sulfuric acid (0.2%).
. Cool overnight, and filter to remove the precipitate. Add 1 mL of 3 N hydrochloric acid, 3 mL of barium chloride TS, and sufficient water to make 50 mL. Mix, and allow to stand for 10 minutes: any turbidity formed is not greater than that produced in a similarly treated control solution containing 0.2 mL of 0.02 N sulfuric acid (0.2%).
Assay—
Standard stock solution—
Transfer about 0.283 g of potassium dichromate, previously dried at 120 for 4 hours and accurately weighed, to a 1000-mL volumetric flask, dissolve in and dilute with water to volume to obtain a solution having a known concentration of about 100 µg of chromium per mL, and mix. Store in a polyethylene bottle.
for 4 hours and accurately weighed, to a 1000-mL volumetric flask, dissolve in and dilute with water to volume to obtain a solution having a known concentration of about 100 µg of chromium per mL, and mix. Store in a polyethylene bottle.
Standard preparations—
Separately transfer 1.0 mL and 2.0 mL of the Standard stock solution to 100-mL volumetric flasks, and transfer 1.5 mL and 2.0 mL of the Standard stock solution to separate 50-mL volumetric flasks. Add 1.0 mL of nitric acid to each flask, dilute the contents of each flask with water to volume, and mix. These Standard preparations contain 1.0, 2.0, 3.0, and 4.0 µg of chromium per mL, respectively.
Assay preparation—
Transfer about 200 mg of Chromium Picolinate, accurately weighed, to a 500-mL volumetric flask, and add 25 mL of water. Slowly add 10 mL of nitric acid, and boil for 10 minutes with constant swirling. Cool the solution, dilute with water to volume, and mix. Filter a portion of the solution, and transfer 5 mL of the filtrate to a 100-mL volumetric flask. Add 1 mL of nitric acid, dilute with water to volume, and mix.
Procedure—
Concomitantly determine the absorbances of the Standard preparations and the Assay preparation at the chromium emission wavelength of 357.9 nm, with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering  851
851 ) equipped with a chromium hollow-cathode lamp and an air–acetylene flame, using diluted nitric acid as the blank. Plot the absorbances of the Standard preparations versus chromium concentration, in µg per mL, and draw the straight line best fitting the four plotted points. From the graph so obtained, determine the chromium concentration, in µg per mL, in the Assay preparation. Calculate the quantity, in mg, of C18H12N3O6Cr in the portion of Chromium Picolinate taken by the formula:
) equipped with a chromium hollow-cathode lamp and an air–acetylene flame, using diluted nitric acid as the blank. Plot the absorbances of the Standard preparations versus chromium concentration, in µg per mL, and draw the straight line best fitting the four plotted points. From the graph so obtained, determine the chromium concentration, in µg per mL, in the Assay preparation. Calculate the quantity, in mg, of C18H12N3O6Cr in the portion of Chromium Picolinate taken by the formula:
(418.31 / 51.996)(10C)
in which C is the concentration, in µg per mL, of chromium in the Assay preparation; 418.31 is the molecular weight of chromium picolinate; and 51.996 is the atomic weight of chromium.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Curtis Phinney
1-301-816-8540 |
(DSN05) Dietary Supplements - Non-Botanicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 982