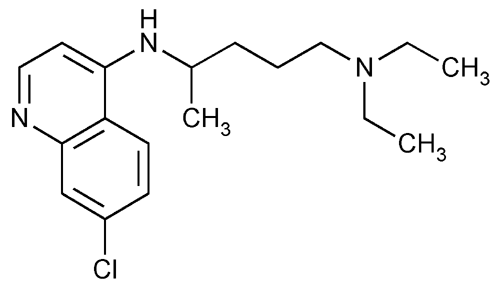
Chloroquine
1,4-Pentanediamine, N4-(7-chloro-4-quinolinyl)-N1,N1-diethyl-.
7-Chloro-4-[[4-(diethylamino)-1-methylbutyl]amino]quinoline
» Chloroquine contains not less than 98.0 percent and not more than 102.0 percent of C18H26ClN3, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Identification—
A:
Dissolve 35 mg in 4 mL of chloroform, and pass through a dry filter: the IR absorption spectrum of the solution so obtained exhibits maxima only at the same wavelengths as that of a solution of USP Chloroquine Phosphate RS prepared as directed in Identification test A under Chloroquine Phosphate.
B: Ultraviolet Absorption  197U
197U —
—
Solution:
10 µg per mL.
Medium:
dilute hydrochloric acid (1 in 1000).
Ratio:
A343 / A329, between 1.00 and 1.15.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 2 hours: it loses not more than 2.0% of its weight.
for 2 hours: it loses not more than 2.0% of its weight.
Residue on ignition  281
281 :
not more than 0.2%.
:
not more than 0.2%.
Change to read:
Assay—
Dissolve about 250 mg of Chloroquine, accurately weighed, in 50 mL of
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientist 1-301-816-8394 |
(MDAA05) Monograph Development-Antivirals and Antimicrobials |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1903
Pharmacopeial Forum: Volume No. 34(1) Page 86
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.