Microcrystalline Cellulose
Cellulose.
Cellulose
» Microcrystalline Cellulose is purified, partially depolymerized cellulose prepared by treating alpha cellulose, obtained as a pulp from fibrous plant material, with mineral acids.
Packaging and storage—
Preserve in tight containers.
Labeling—
The labeling indicates the nominal loss on drying, bulk density, and degree of polymerization values. Degree of polymerization compliance is determined using Identification test B. Where the particle size distribution is stated in the labeling, proceed as directed under Particle size distribution. The labeling indicates with which technique the particle size distribution was determined if a technique other than analytical sieving was used; and the labeling indicates the d10, d50, and d90 values and the range for each.
Identification—
A:
Prepare iodinated zinc chloride solution by dissolving 20 g of zinc chloride and 6.5 g of potassium iodide in 10.5 mL of water. Add 0.5 g of iodine, and shake for 15 minutes. Place about 10 mg of Microcrystalline Cellulose on a watch glass, and disperse in 2 mL of iodinated zinc chloride solution: the substance takes on a violet-blue color.
B:
Transfer 1.3 g of Microcrystalline Cellulose, accurately weighed to 0.1 mg, to a 125-mL conical flask. Add 25.0 mL of water and 25.0 mL of 1.0 M cupriethylenediamine hydroxide solution. Immediately purge the solution with nitrogen, insert the stopper, and shake on a wrist action shaker, or other suitable mechanical shaker, until completely dissolved. Transfer an appropriate volume of the solution to a calibrated number 150 Cannon-Fenske, or equivalent, viscosimeter. Allow the solution to equilibrate at 25 ± 0.1 for not less than 5 minutes. Time the flow between the two marks on the viscosimeter, and record the flow time, t1, in seconds. Calculate the kinematic viscosity, (KV)1, of the Microcrystalline Cellulose taken by the formula:
for not less than 5 minutes. Time the flow between the two marks on the viscosimeter, and record the flow time, t1, in seconds. Calculate the kinematic viscosity, (KV)1, of the Microcrystalline Cellulose taken by the formula:
 911
911 ). Obtain the flow time, t2, for a 0.5 M cupriethylenediamine hydroxide solution using a number 100 Cannon-Fenske, or equivalent, viscosimeter. Calculate the kinematic viscosity, (KV)2, of the solvent by the formula:
). Obtain the flow time, t2, for a 0.5 M cupriethylenediamine hydroxide solution using a number 100 Cannon-Fenske, or equivalent, viscosimeter. Calculate the kinematic viscosity, (KV)2, of the solvent by the formula:
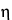 rel, of the Microcrystalline Cellulose specimen taken by the formula:
rel, of the Microcrystalline Cellulose specimen taken by the formula:
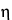 ]c, by interpolation, using the Intrinsic Viscosity Table in the Reference Tables section. Calculate the degree of polymerization, P, by the formula:
]c, by interpolation, using the Intrinsic Viscosity Table in the Reference Tables section. Calculate the degree of polymerization, P, by the formula:
t1(k1)
in which k1 is the viscosimeter constant (see Viscosity
t2(k2)
in which k2 is the viscosimeter constant. Determine the relative viscosity, (KV)1 / (KV)2
Determine the intrinsic viscosity, [(95)[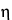 ]c /WS[(l00 – %LOD)/100]
]c /WS[(l00 – %LOD)/100]
in which WS is the weight, in g, of the Microcrystalline Cellulose taken; and %LOD is the value obtained from the test for Loss on drying. The degree of polymerization is not greater than 350.
Microbial enumeration tests  61
61 and Tests for specified microorganisms
and Tests for specified microorganisms  62
62 —
The total aerobic microbial count does not exceed 1000 cfu per g, the total combined molds and yeasts count does not exceed 100 cfu per g, and it meets the requirements of the tests for absence of Staphylococcus aureus and Pseudomonas aeruginosa and for absence of Escherichia coli and Salmonella species.
—
The total aerobic microbial count does not exceed 1000 cfu per g, the total combined molds and yeasts count does not exceed 100 cfu per g, and it meets the requirements of the tests for absence of Staphylococcus aureus and Pseudomonas aeruginosa and for absence of Escherichia coli and Salmonella species.
Conductivity—
Shake about 5 g with 40 mL of water for 20 minutes, and centrifuge. Retain the supernatant for use in the pH test. Using an appropriate conductivity meter that has been standardized with a potassium chloride conductivity calibration standard having a conductivity of 100 µS per cm, measure the conductivity of the supernatant after a stable reading is obtained, and measure the conductivity of the water used to prepare the test specimen. The conductivity of the supernatant does not exceed the conductivity of the water by more than 75 µS per cm.
pH  791
791 :
between 5.0 and 7.5 in the supernatant obtained in the Conductivity test.
:
between 5.0 and 7.5 in the supernatant obtained in the Conductivity test.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 3 hours: it loses not more than 7.0% of its weight, or some other lower percentage, or is within a percentage range, as specified in the labeling.
for 3 hours: it loses not more than 7.0% of its weight, or some other lower percentage, or is within a percentage range, as specified in the labeling.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Bulk density—
Use a volumeter that has been fitted with a 10-mesh screen. The volumeter is freestanding of the brass or stainless steel cup, which is calibrated to a capacity of 25.0 ± 0.05 mL and has an inside diameter of 30.0 ± 2.0 mm. Weigh the empty cup, position it under the chute, and slowly pour the powder from a height of 5.1 cm (2 inches) above the funnel through the volumeter, at a rate suitable to prevent clogging, until the cup overflows. [note—If excessive clogging of the screen occurs, remove the screen.] Level the excess powder, and weigh the filled cup. Calculate the bulk density by dividing the weight of the powder in the cup by the volume of the cup: the bulk density is within the labeled specification.
Particle size distribution—
[note—In cases where there are no functionality-related concerns regarding the particle size distribution of the article, this test may be omitted.] Where the labeling states the particle size distribution, determine the particle size distribution as directed in Particle Size Distribution Estimation by Analytical Sieving  786
786 , or by a suitable validated procedure.
, or by a suitable validated procedure.
Water-soluble substances—
Shake 5.0 g with about 80 mL of water for 10 minutes, filter with the aid of vacuum through filter paper (Whatman No. 42 or equivalent) into a vacuum flask. Transfer the filtrate to a tared beaker, evaporate to dryness without charring, dry at 105 for 1 hour, cool in a desiccator, and weigh: the difference between the weight of the residue and the weight obtained from a blank determination does not exceed 12.5 mg (0.25%).
for 1 hour, cool in a desiccator, and weigh: the difference between the weight of the residue and the weight obtained from a blank determination does not exceed 12.5 mg (0.25%).
Ether-soluble substances—
Place 10.0 g in a chromatographic column having an internal diameter of about 20 mm, and pass 50 mL of peroxide-free ether through the column. Evaporate the eluate to dryness in a previously dried and tared evaporating dish with the aid of a current of air in a fume hood. After all the ether has evaporated, dry the residue at 105 for 30 minutes, cool in a desiccator, and weigh: the difference between the weight of the residue and the weight obtained from a blank determination does not exceed 5.0 mg (0.05%).
for 30 minutes, cool in a desiccator, and weigh: the difference between the weight of the residue and the weight obtained from a blank determination does not exceed 5.0 mg (0.05%).
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Kevin T. Moore, Ph.D.
Scientist 1-301-816-8369 |
(EM205) Excipient Monographs 2 |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 1199
Pharmacopeial Forum: Volume No. 31(5) Page 1421
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.