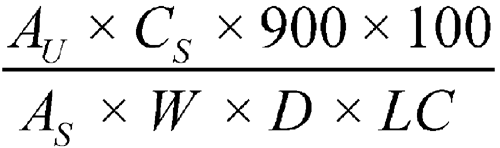Cefadroxil for Oral Suspension
» Cefadroxil for Oral Suspension is a dry mixture of Cefadroxil and one or more suitable buffers, colors, diluents, and flavors. It contains the equivalent of not less than 90.0 percent and not more than 120.0 percent of the labeled amount of C16H17N3O5S.
Packaging and storage—
Preserve in tight containers.
Identification—
Constitute 1 container of Cefadroxil for Oral Suspension as directed in the labeling. Mix a portion of the resulting suspension with water to obtain a concentration of about 2 mg of cefadroxil per mL, and filter: the filtrate so obtained responds to Identification test B under Cefadroxil.
Dissolution  711
711 —
—
Medium:
water; 900 mL.
Apparatus 2:
25 rpm.
Time:
30 minutes.
Procedure—
Accurately weigh 5.0 mL of the constituted Oral Suspension, and transfer to the dissolution vessel. Determine the amount of cefadroxil dissolved by employing UV absorption at the wavelength of maximum absorbance at about 263 nm on filtered portions of the solution under test, suitably diluted with Medium, if necessary, in comparison with a Standard solution having a known concentration of USP Cefadroxil RS in the same Medium. Calculate the amount of cefadroxil dissolved by the formula:
in which AU and AS are the absorbances obtained from the solution under test and the Standard solution, respectively; CS is the concentration, in mg per mL, of the Standard solution; 900 is the volume, in mL, of Medium; 100 is the conversion factor to percentage; W is the weight, in mg, of the 5 mL of constituted Oral Suspension taken; D is the dilution factor of the solution under test; and LC is the label claim, in mg per 5 mL.
Tolerances—
Not less than 75% (Q) of the labeled amount of cefadroxil is dissolved in 30 minutes.
Uniformity of dosage units  905
905 —
—
For solid packaged in single-unit containers:
meets the requirements.
Deliverable volume  698
698 :
meets the requirements.
:
meets the requirements.
pH  791
791 :
between 4.5 and 6.0, in the suspension constituted as directed in the labeling.
:
between 4.5 and 6.0, in the suspension constituted as directed in the labeling.
Water, Method I  921
921 :
not more than 2.0%, except where it is labeled as containing 100 mg of cefadroxil per mL after constitution, the limit is not more than 3.0%.
:
not more than 2.0%, except where it is labeled as containing 100 mg of cefadroxil per mL after constitution, the limit is not more than 3.0%.
Assay—
pH 5.0 Buffer, Mobile phase, Standard preparation, and Chromatographic system—
Proceed as directed in the Assay under Cefadroxil.
Assay preparation—
Constitute a container of Cefadroxil for Oral Suspension as directed in the labeling. Transfer an accurately measured volume of the oral suspension thus obtained, equivalent to about 250 mg of cefadroxil, to a 250-mL volumetric flask, dilute with pH 5.0 buffer to volume, and stir by mechanical means for 5 minutes. Pass about 25 mL of the resulting solution through a suitable filter of 0.8 µm or finer porosity, and use the clear filtrate as the Assay preparation. Use this solution on the day prepared.
Procedure—
Proceed as directed for Procedure in the Assay under Cefadroxil. Calculate the quantity, in mg, of C16H17N3O5S in each mL of Cefadroxil for Oral Suspension taken by the formula:
0.25(CE / V)(rU / rS)
in which V is the volume, in mL, of Cefadroxil for Oral Suspension taken, and the other terms are as defined therein.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ahalya Wise, M.S.
Scientist 1-301-816-8161 |
(MDANT05) Monograph Development-Antibiotics |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Margareth R.C. Marques, Ph.D.
Senior Scientist 1-301-816-8106 |
(BPC05) Biopharmaceutics05 |
USP32–NF27 Page 1821
Pharmacopeial Forum: Volume No. 32(2) Page 315
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.