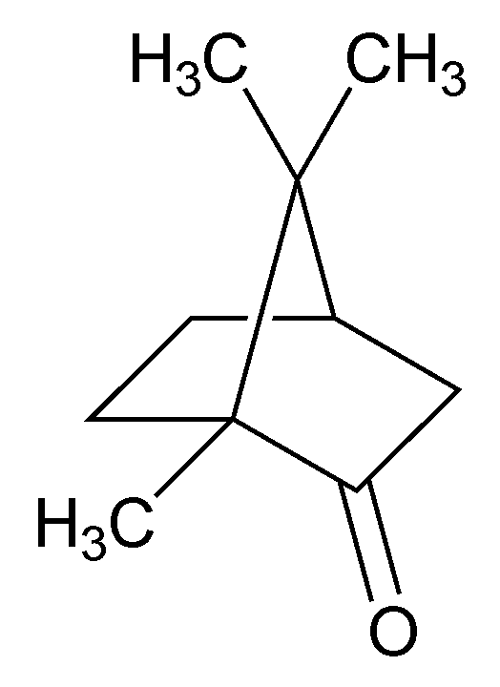
Camphor
» Camphor is a ketone obtained from Cinnamomum camphora (Linné) Nees et Ebermaier (Fam. Lauraceae) (Natural Camphor) or produced synthetically (Synthetic Camphor).
Packaging and storage—
Preserve in tight containers, and avoid exposure to excessive heat.
Labeling—
Label it to indicate whether it is obtained from natural sources or is prepared synthetically.
Specific rotation  781S
781S :
between +41
:
between +41 and +43
and +43 , for natural Camphor.
, for natural Camphor.
Test solution:
100 mg per mL, in alcohol.
Synthetic Camphor is optically inactive.
Appearance of solution—
A 1 in 10 solution in solvent hexane is clear.
Limit of nonvolatile residue—
Heat 2.0 g in a tared dish on a steam bath until sublimation is complete. Then dry the residue at 120 for 3 hours, cool, and weigh: the weight of the residue does not exceed 1.0 mg (0.05%).
for 3 hours, cool, and weigh: the weight of the residue does not exceed 1.0 mg (0.05%).
Halogens—
Mix 100 mg of finely divided Camphor with 200 mg of sodium peroxide in a clean, dry, hard glass test tube of about 25-mm internal diameter and 20-cm length. Suspend the tube at an angle of about 45 by means of a clamp placed at the upper end, and gently heat the tube, starting near the upper end, but not heating the clamp, and gradually bringing the heat toward the lower part of the tube until incineration is complete. Dissolve the residue in 25 mL of warm water, acidify with nitric acid, and filter the solution into a comparison tube. Wash the test tube and the filter with two 10-mL portions of hot water, adding the washings to the filtered solution. To the filtrate add 0.50 mL of 0.10 N silver nitrate, dilute with water to 50 mL, and mix: the turbidity does not exceed that produced in a blank test with the same quantities of the same reagents and 0.050 mL of 0.020 N hydrochloric acid (0.035%).
by means of a clamp placed at the upper end, and gently heat the tube, starting near the upper end, but not heating the clamp, and gradually bringing the heat toward the lower part of the tube until incineration is complete. Dissolve the residue in 25 mL of warm water, acidify with nitric acid, and filter the solution into a comparison tube. Wash the test tube and the filter with two 10-mL portions of hot water, adding the washings to the filtered solution. To the filtrate add 0.50 mL of 0.10 N silver nitrate, dilute with water to 50 mL, and mix: the turbidity does not exceed that produced in a blank test with the same quantities of the same reagents and 0.050 mL of 0.020 N hydrochloric acid (0.035%).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientist 1-301-816-8394 |
(MDAA05) Monograph Development-Antivirals and Antimicrobials |
USP32–NF27 Page 1773
Pharmacopeial Forum: Volume No. 31(3) Page 742