- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Butylated Hydroxytoluene |
 |
(Butylhydroxytoluene, Ph Eur monograph 0581)
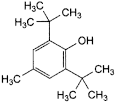
C15H24O 220.4 128-37-0
Antioxidant.
Ph Eur
Butylhydroxytoluene is 2,6-bis(1,1-dimethylethyl)-4-methylphenol.
A white or yellowish-white, crystalline powder, practically insoluble in water, very soluble in acetone, freely soluble in alcohol and in vegetable oils.
First identification A, C.
Second identification
A, B, D.
A. Freezing-point (see Tests).
B. Dissolve 0.500 g in ethanol R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of this solution to 100.0 mL with ethanol R. Examined between 230 nm and 300 nm (2.2.25), the solution shows an absorption maximum at 278 nm. The specific absorbance at the maximum is 80 to 90.
C. Examine by infrared absorption spectrophotometry (2.2.24), comparing with the spectrum obtained with butylhydroxytoluene CRS.
D. Dissolve about 10 mg in 2 mL of alcohol R. Add 1 mL of a 1 g/L solution of testosterone propionate R in alcohol R and 2 mL of dilute sodium hydroxide solution R. Heat in a water-bath at 80 °C for 10 min and allow to cool. A blue colour develops.
Dissolve 1.0 g in methanol R and dilute to 10 mL with the same solvent. The solution is clear (2.2.1) and not more intensely coloured than reference solution Y5 or BY5 (2.2.2, Method II).
69 °C to 70 °C.
Examine by thin-layer chromatography (2.2.27), using silica gel G R as the coating substance.
Test solution Dissolve 0.2 g of the substance to be examined in methanol R and dilute to 10.0 mL with the same solvent.
Reference solution Dilute 1 mL of the test solution to 200 mL with methanol R.
Apply separately to the plate 10 µL of each solution. Develop over a path of 15 cm using methylene chloride R. Dry the plate in air and spray with a freshly prepared mixture of 10 volumes of potassium ferricyanide solution R, 20 volumes of ferric chloride solution R1 and 70 volumes of water R. Any spot in the chromatogram obtained with the test solution, apart from the principal spot, is not more intense than the spot in the chromatogram obtained with the reference solution (0.5 per cent).
Not more than 0.1 per cent, determined on 1.0 g.
Ph Eur

