- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Colecalciferol Concentrate (Oily Form) |
 |
(Cholecalciferol Concentrate (Oily Form), Ph Eur monograph 0575)
Vitamin D analogue (Vitamin D3).
Ph Eur
Solution of Cholecalciferol (0072) in a suitable vegetable fatty oil, authorised by the competent authority.
90.0 per cent to 110.0 per cent of the cholecalciferol content stated on the label, which is not less than 500 000 IU/g.
It may contain suitable stabilisers such as antioxidants.
Clear, yellow liquid.
Practically insoluble in water, slightly soluble in anhydrous ethanol, miscible with solvents of fats.
Partial solidification may occur, depending on the temperature.
First identification A, C.
Second identification A, B.
A. Thin-layer chromatography (2.2.27). Prepare the solutions immediately before use.
Test solution Dissolve an amount of the preparation to be examined corresponding to 400 000 IU in ethylene chloride R containing 10 g/L of squalane R and 0.1 g/L of butylhydroxytoluene R and dilute to 4 mL with the same solution.
Reference solution (a) Dissolve 10 mg of cholecalciferol CRS in ethylene chloride R containing 10 g/L of squalane R and 0.1 g/L of butylhydroxytoluene R and dilute to 4 mL with the same solution.
Reference solution (b) Dissolve 10 mg of ergocalciferol CRS in ethylene chloride R containing 10 g/L of squalane R and 0.1 g/L of butylhydroxytoluene R and dilute to 4 mL with the same solution.
Plate TLC silica gel G plate R.
Mobile phase A 0.1 g/L solution of butylhydroxytoluene R in a mixture of equal volumes of cyclohexane R and peroxide-free ether R.
Application 20 µL.
Development Immediately, protected from light, over a path of 15 cm.
Drying In air.
Detection Spray with sulfuric acid R.
Results The chromatogram obtained with the test solution shows immediately a bright yellow principal spot which rapidly becomes orange-brown, then gradually greenish-grey, remaining so for 10 min. This spot is similar in position, colour and size to the spot in the chromatogram obtained with reference solution (a). The chromatogram obtained with reference solution (b) shows immediately at the same level an orange principal spot which gradually becomes reddish-brown and remains so for 10 min.
B. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution Prepare a solution in cyclohexane R containing the equivalent of about 400 IU/mL.
Spectral range 250-300 nm.
Absorption maximum At 267 nm.
C. Examine the chromatograms obtained in the assay.
Results The principal peak in the chromatogram obtained with the test solution is similar in retention time to the principal peak in the chromatogram obtained with reference solution (a).
Maximum 2.0.
Dissolve 5.0 g in 25 mL of the prescribed mixture of solvents.
Maximum 20.
The thresholds indicated under Related substances (Table 2034.-1) in the general monograph Substances for pharmaceutical use (2034) do not apply.
Carry out the assay as rapidly as possible, avoiding exposure to actinic light and air.
Liquid chromatography (2.2.29).
Test solution Dissolve a quantity of the preparation to be examined, weighed with an accuracy of 0.1 per cent, equivalent to about 400 000 IU, in 10.0 mL of toluene R and dilute to 100.0 mL with the mobile phase.
Reference solution (a) Dissolve 10.0 mg of cholecalciferol CRS without heating in 10.0 mL of toluene R and dilute to 100.0 mL with the mobile phase.
Reference solution (b) Dilute 1.0 mL of cholecalciferol for system suitability CRS to 5.0 mL with the mobile phase. Heat in a water-bath at 90 °C under a reflux condenser for 45 min and cool.
Reference solution (c) Dissolve 0.10 g of cholecalciferol CRS without heating in toluene R and dilute to 100.0 mL with the same solvent.
Reference solution (d) Dilute 5.0 mL of reference solution (c) to 50.0 mL with the mobile phase. Keep the solution in iced water.
Reference solution (e) Place 5.0 mL of reference solution (c) in a volumetric flask, add about 10 mg of butylhydroxytoluene R and displace air from the flask with nitrogen R. Heat in a water-bath at 90 °C under a reflux condenser protected from light and under nitrogen R for 45 min. Cool and dilute to 50.0 mL with the mobile phase.
- — size: l = 0.25 m, Ø = 4.6 mm;
- — stationary phase: silica gel for chromatography R (5 µm).
Mobile phase pentanol R, hexane R (3:997 V/V).
Flow rate 2 mL/min.
Detection Spectrophotometer at 254 nm.
Injection The chosen volume of each solution (the same volume for reference solution (a) and for the test solution); automatic injection device or sample loop recommended.
Relative retention With reference to cholecalciferol: pre-cholecalciferol = about 0.4; trans-cholecalciferol = about 0.5.
System suitability Reference solution (b):
- — resolution: minimum 1.0 between the peaks due to pre-cholecalciferol and trans-cholecalciferol; if necessary adjust the proportions of the constituents and the flow rate of the mobile phase to obtain this resolution;
- — repeatability: maximum relative standard deviation of 1.0 per cent for the peak due to cholecalciferol after 6 injections.
Calculate the conversion factor (f) using the following expression:
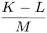
K |
= |
area (or height) of the peak due to cholecalciferol in the chromatogram obtained with reference solution (d); |
L |
= |
area (or height) of the peak due to cholecalciferol in the chromatogram obtained with reference solution (e); |
M |
= |
area (or height) of the peak due to pre-cholecalciferol in the chromatogram obtained with reference solution (e). |
The value of f determined in duplicate on different days may be used during the entire procedure.
Calculate the content of cholecalciferol in International Units per gram using the following expression:
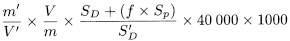
m |
= |
mass of the preparation to be examined in the test solution, in milligrams; |
m ′ |
= |
mass of cholecalciferol CRS in reference solution (a), in milligrams; |
V |
= |
volume of the test solution (100 ml); |
V ′ |
= |
volume of reference solution (a) (100 ml); |
S D |
= |
area (or height) of the peak due to cholecalciferol in the chromatogram obtained with the test solution; |
S ′ D |
= |
area (or height) of the peak due to cholecalciferol in the chromatogram obtained with reference solution (a); |
S p |
= |
area (or height) of the peak due to pre-cholecalciferol in the chromatogram obtained with the test solution; |
f |
= |
conversion factor. |
In an airtight, well-filled container, protected from light. The contents of an opened container are to be used as soon as possible; any unused part is to be protected by an atmosphere of nitrogen.
The label states:
- — the number of International Units per gram;
- — the method of restoring the solution if partial solidification occurs.
Ph Eur

