- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Diclofenac Potassium |
 |
(Ph. Eur. monograph 1508)
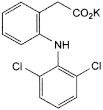
C14H10Cl2KNO2 334.2 15307-81-0
Cyclo-oxygenase inhibitor; analgesic; anti-inflammatory.
Ph Eur
Potassium [2-[(2,6-dichlorophenyl)amino]phenyl]acetate.
99.0 per cent to 101.0 per cent (dried substance).
White or slightly yellowish, slightly hygroscopic, crystalline powder.
Sparingly soluble in water, freely soluble in methanol, soluble in ethanol (96 per cent), slightly soluble in acetone.
First identification A, D.
Second identification B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Preparation Discs.
Comparison diclofenac potassium CRS.
B. Thin-layer chromatography (2.2.27).
Test solution Dissolve 25 mg of the substance to be examined in methanol R and dilute to 5 mL with the same solvent.
Reference solution (a) Dissolve 25 mg of diclofenac potassium CRS in methanol R and dilute to 5 mL with the same solvent.
Reference solution (b) Dissolve 10 mg of indometacin R in reference solution (a) and dilute to 2 mL with the same solution.
Plate TLC silica gel GF 254 plate R.
Mobile phase concentrated ammonia R, methanol R, ethyl acetate R (10:10:80 V/V/V).
Application 5 µL.
Development Over a path of 10 cm.
Drying In air.
Detection Examine in ultraviolet light at 254 nm.
System suitability Reference solution (b):
- — the chromatogram shows 2 clearly separated spots.
Results The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
C. Dissolve about 10 mg in 10 mL of ethanol (96 per cent) R. To 1 mL of this solution add 0.2 mL of a mixture, prepared immediately before use, of equal volumes of a 6 g/L solution of potassium ferricyanide R and a 9 g/L solution of ferric chloride R. Allow to stand protected from light for 5 min. Add 3 mL of a 10 g/L solution of hydrochloric acid R. Allow to stand protected from light for 15 min. A blue colour develops and a precipitate is formed.
D. Suspend 0.5 g in 10 mL of water R. Stir and add water R until the substance is dissolved. Add 2 mL of hydrochloric acid R1, stir for 1 h and filter with the aid of vacuum. Neutralise with sodium hydroxide solution R. The solution gives reaction (b) of potassium (2.3.1).
The solution is clear (2.2.1) and its absorbance (2.2.25) at 440 nm is not greater than 0.05.
Dissolve 1.25 g in methanol R and dilute to 25.0 mL with the same solvent.
Liquid chromatography (2.2.29).
Test solution Dissolve 50.0 mg of the substance to be examined in methanol R and dilute to 50.0 mL with the same solvent.
Reference solution (a) Dilute 2.0 mL of the test solution to 100.0 mL with methanol R. Dilute 1.0 mL of this solution to 10.0 mL with methanol R.
Reference solution (b) Dilute 1.0 mL of the test solution to 200.0 mL with methanol R. In 1.0 mL of this solution dissolve the contents of a vial of diclofenac impurity A CRS.
- — size: l = 0.25 m, Ø = 4.6 mm;
- — stationary phase: end-capped octylsilyl silica gel for chromatography R (5 µm).
Mobile phase Mix 34 volumes of a solution containing 0.5 g/L of phosphoric acid R and 0.8 g/L of sodium dihydrogen phosphate R, adjusted to pH 2.5 with phosphoric acid R, and 66 volumes of methanol R.
Flow rate 1 mL/min.
Detection Spectrophotometer at 254 nm.
Injection 20 µL.
Run time 1.5 times the retention time of diclofenac.
Retention time Impurity A = about 12 min; diclofenac = about 25 min.
System suitability Reference solution (b):
- — resolution: minimum 6.5 between the peaks due to impurity A and diclofenac.
- — impurities A, B, C, D, E: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent);
- — total: not more than 2.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent);
- — disregard limit: 0.25 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Maximum 10 ppm.
2.0 g complies with test C. Use a quartz crucible. Prepare the reference solution using 2 mL of lead standard solution (10 ppm Pb) R.
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C for 3 h.
Dissolve 0.250 g in 30 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 33.42 mg of C14H10Cl2KNO2.
In an airtight container, protected from light.
Specified impurities A, B, C, D, E.
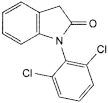
A. 1-(2,6-dichlorophenyl)-1,3-dihydro-2H-indol-2-one,
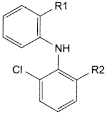
B. R1 = CHO, R2 = Cl: 2-[(2,6-dichlorophenyl)amino]benzaldehyde,
C. R1 = CH2OH, R2 = Cl: [2-[(2,6-dichlorophenyl)amino]phenyl]methanol,
D. R1 = CH2-CO2H, R2 = Br: 2-[2-[(2-bromo-6-chlorophenyl)amino]phenyl]acetic acid,
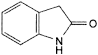
E. 1,3-dihydro-2H-indol-2-one.
Ph Eur

