- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Levodopa |
 |
(Ph. Eur. monograph 0038)
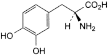
C9H11NO4 197.2 59-92-7
Dopamine precursor; treatment of Parkinson's disease.
Dispersible Co-beneldopa Tablets
When l-dopa is prescribed or demanded, Levodopa shall be dispensed or supplied.
Ph Eur
(2S)-2-Amino-3-(3,4-dihydroxyphenyl)propanoic acid.
99.0 per cent to 101.0 per cent (dried substance).
White or almost white, crystalline powder.
Slightly soluble in water, practically insoluble in ethanol (96 per cent). It is freely soluble in 1 M hydrochloric acid and sparingly soluble in 0.1 M hydrochloric acid.
Infrared absorption spectrophotometry (2.2.24).
Comparison levodopa CRS.
The solution is not more intensely coloured than reference solution BY6 (2.2.2, Method II).
Dissolve 1.0 g in a 103 g/L solution of hydrochloric acid R and dilute to 25 mL with the same solution.
4.5 to 7.0.
Shake 0.10 g with 10 mL of carbon dioxide-free water R for 15 min.
Liquid chromatography (2.2.29). Use freshly prepared solutions.
Solution A 10.3 g/L solution of hydrochloric acid R.
Test solution Dissolve 0.100 g of the substance to be examined in solution A and dilute to 25 mL with solution A.
Reference solution (a) Dilute 1.0 mL of the test solution to 50.0 mL with solution A. Dilute 5.0 mL of this solution to 100.0 mL with solution A.
Reference solution (b) Dissolve 8 mg of tyrosine R (impurity B) and 4 mg of 3-methoxy-l-tyrosine R (l-isomer of impurity C) in 2 mL of the test solution and dilute to 50 mL with solution A. Dilute 5 mL of this solution to 100 mL with solution A.
- — size: l = 0.25 m, Ø = 4.6 mm;
- — stationary phase: spherical di-isobutyloctadecylsilyl silica gel for chromatography R (5 µm) with a pore size of 8 nm.
- — mobile phase A: 0.1 M phosphate buffer solution pH 3.0 R;
- — mobile phase B: methanol R, 0.1 M phosphate buffer solution pH 3.0 R (18:85 V/V);

Flow rate 1 mL/min.
Detection Spectrophotometer at 280 nm.
Injection 20 µL.
Identification of impurities Use the chromatogram obtained with reference solution (b) to identify the peaks due to impurities B and C.
Relative retention With reference to levodopa (retention time = about 6 min): impurity A = about 0.7; impurity B = about 2; impurity C = about 3.5.
System suitability Reference solution (b):
- — resolution: minimum 10 between the peaks due to levodopa and impurity B.
- — correction factor: for the calculation of content, multiply the peak area of impurity B by 2.2;
- — impurity B: not more than 5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent);
- — impurity C: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent);
- — impurity A: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent);
- — unspecified impurities: for each impurity, not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent);
- — total: not more than 10 times the area of the principal peak in the chromatogram obtained with reference solution (a) (1.0 per cent);
- — disregard limit: 0.3 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.03 per cent).
Liquid chromatography (2.2.29). Use freshly prepared solutions.
Test solution Dissolve 25 mg of the substance to be examined in the mobile phase and dilute to 25 mL with the mobile phase.
Reference solution (a) Dilute 5.0 mL of the test solution to 20.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 50.0 mL with the mobile phase.
Reference solution (b) Dissolve 10 mg of d-dopa R (impurity D) in 10 mL of the test solution. Dilute 1 mL of this solution to 100 mL with the mobile phase.
- — size: l = 0.15 m, Ø = 3.9 mm;
- — stationary phase: spherical end-capped octadecylsilyl silica gel for chromatography R (5 µm).
Mobile phase Dissolve separately 200 mg of copper acetate R and 387 mg of N,N-dimethyl-l-phenylalanine R in 250 mL of water R; mix the 2 solutions and adjust immediately to pH 4.0 with acetic acid R; add 50 mL of methanol R and dilute to 1000 mL with water R; mix and filter.
Flow rate 1 mL/min.
Detection Spectrophotometer at 280 nm.
Injection 20 µL.
Run time Twice the retention time of levodopa.
Relative retention With reference to levodopa (retention time = about 7 min): impurity D = about 0.4.
System suitability Reference solution (b):
- — resolution: minimum 5 between the peaks due to impurity D and levodopa.
Limit:
- — impurity D: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent).
Maximum 10 ppm.
2.0 g complies with test C. Prepare the reference solution using 2 mL of lead standard solution (10 ppm Pb) R.
Maximum 1.0 per cent, determined on 0.500 g by drying in an oven at 105 °C.
Maximum 0.1 per cent, determined on 1.0 g.
Dissolve 0.150 g, heating if necessary, in 5 mL of anhydrous formic acid R. Add 50 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 19.72 mg of C9H11NO4.
Protected from light.
Specified impurities A, B, C, D.
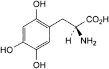
A. (2S)-2-amino-3-(2,4,5-trihydroxyphenyl)propanoic acid,
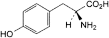
B. (2S)-2-amino-3-(4-hydroxyphenyl)propanoic acid (tyrosine),
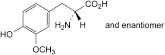
C. (2RS)-2-amino-3-(4-hydroxy-3-methoxyphenyl)propanoic acid (3-methoxy-dl-tyrosine),
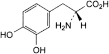
D. (2R)-2-amino-3-(3,4-dihydroxyphenyl)propanoic acid (d-dopa).
Ph Eur

