- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Neomycin Sulfate |
 |
Neomycin Sulphate
(Ph. Eur. monograph 0197)

C23H46N6O13,xH2SO4 615 (base)
Aminoglycoside antibacterial.
Dexamethasone and Neomycin Ear Spray
Hydrocortisone and Neomycin Cream
Hydrocortisone Acetate and Neomycin Ear Drops
Hydrocortisone Acetate and Neomycin Eye Drops
Hydrocortisone Acetate and Neomycin Eye Ointment
Ph Eur
Mixture of sulfates of substances produced by the growth of certain selected strains of Streptomyces fradiae, the main component being the sulfate of 2-deoxy-4-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-5-O-[3-O-(2,6-diamino-2,6-dideoxy-β-l-idopyranosyl)-β-d-ribofuranosyl]-d-streptamine (neomycin B).
Minimum of 680 IU/mg (dried substance).
White or yellowish-white powder, hygroscopic.
Very soluble in water, very slightly soluble in alcohol, practically insoluble in acetone.
A. Examine the chromatograms obtained in the test for related substances.
- — the retention time of the principal peak in the chromatogram obtained with the test solution is approximately the same as that of the principal peak in the chromatogram obtained with reference solution (e),
- — it complies with the limits given for impurity C.
B. It gives reaction (a) of sulfates (2.3.1).
5.0 to 7.5.
Dissolve 0.1 g in carbon dioxide-free water R and dilute to 10 mL with the same solvent.
+ 53.5 to + 59.0 (dried substance).
Dissolve 1.00 g in water R and dilute to 10.0 mL with the same solvent.
Liquid chromatography (2.2.29).
Test solution Dissolve 25.0 mg of the substance to be examined in the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution (a) Dissolve 25.0 mg of framycetin sulfate CRS in the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution (b) Dilute 5.0 mL of reference solution (a) to 100.0 mL with the mobile phase.
Reference solution (c) Dilute 1.0 mL of reference solution (a) to 100.0 mL with the mobile phase.
Reference solution (d) Dissolve the contents of a vial of neamine CRS (corresponding to 0.5 mg) in the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution (e) Dissolve 10 mg of neomycin sulfate CRS in the mobile phase and dilute to 100.0 mL with the mobile phase.
- — size: l = 0.25 m, Ø = 4.6 mm,
- — stationary phase: base-deactivated octadecylsilyl silica gel for chromatography R (5 µm),
- — temperature: 25 °C.
Mobile phase Mix 20.0 mL of trifluoroacetic acid R, 6.0 mL of carbonate-free sodium hydroxide solution R and 500 mL of water R, allow to equilibrate, dilute to 1000 mL with water R and degas.
Flow rate 0.7 mL/min.
Post-column solution Carbonate-free sodium hydroxide solution R diluted 1 in 25 previously degassed, which is added pulse-less to the column effluent using a 375 µL polymeric mixing coil.
Flow rate 0.5 mL/min.
Detection Pulsed amperometric detector with a gold indicator electrode, a silver-silver chloride reference electrode and a stainless steel auxiliary electrode which is the cell body, held at respectively 0.00 V detection, + 0.80 V oxidation and - 0.60 V reduction potentials, with pulse durations according to the instrument used.
Injection 10 µL; inject the test solution and the reference solutions (b), (c), (d) and (e).
Run time 1.5 times the retention time of neomycin B.
Relative retention With reference to neomycin B (retention time = about 10 min): impurity A = about 0.65; impurity C = about 0.9; impurity G = about 1.1.
- — resolution: minimum of 2.0 between the peaks due to impurity C and to neomycin B in the chromatogram obtained with reference solution (e); if necessary, adjust the volume of the carbonate-free sodium hydroxide solution in the mobile phase,
- — signal-to-noise ratio: minimum 10 for the principal peak in the chromatogram obtained with reference solution (c).
- — impurity A: not more than the area of the principal peak in the chromatogram obtained with reference solution (d) (2.0 per cent),
- — impurity C: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (b) (15.0 per cent) and not less than 0.6 times the area of the principal peak in the chromatogram obtained with reference solution (b) (3.0 per cent),
- — any other impurity: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (5.0 per cent),
- — total of other impurities: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (b) (15.0 per cent),
- — disregard limit: area of the principal peak in the chromatogram obtained with reference solution (c) (1.0 per cent).
27.0 per cent to 31.0 per cent (dried substance).
Dissolve 0.250 g in 100 mL of water R and adjust the solution to pH 11 using concentrated ammonia R. Add 10.0 mL of 0.1 M barium chloride and about 0.5 mg of phthalein purple R. Titrate with 0.1 M sodium edetate adding 50 mL of alcohol R when the colour of the solution begins to change, continuing the titration until the violet-blue colour disappears.
1 mL of 0.1 M barium chloride is equivalent to 9.606 mg of SO4.
Maximum 8.0 per cent, determined on 1.000 g by drying at 60 °C over diphosphorus pentoxide R at a pressure not exceeding 0.7 kPa for 3 h.
Maximum 1.0 per cent, determined on 1.0 g.
Carry out the microbiological assay of antibiotics (2.7.2).
In an airtight container, protected from light.
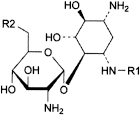
A. R1 = H, R2 = NH2: 2-deoxy-4-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-d-streptamine (neamine or neomycin A-LP),
B. R1 = CO-CH3, R2 = NH2: 3-N-acetyl-2-deoxy-4-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-d-streptamine (3-acetylneamine),
D. R1 = H, R2 = OH: 4-O-(2-amino-2-deoxy-α-d-glucopyranosyl)-2-deoxy-d-streptamine (paromamine or neomycin D),
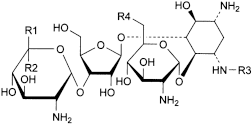
C. R1 = CH2-NH2, R2 = R3 = H, R4 = NH2: 2-deoxy-4-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-5-O-[3-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-β-d-ribofuranosyl]-d-streptamine (neomycin C),
E. R1 = R3 = H, R2 = CH2-NH2, R4 = OH: 4-O-(2-amino-2-deoxy-α-d-glucopyranosyl)-2-deoxy-5-O-[3-O-(2,6-diamino-2,6-dideoxy-β-l-idopyranosyl)-β-d-ribofuranosyl]-d-streptamine (paromomycin I or neomycin E),
F. R1 = CH2-NH2, R2 = R3 = H, R4 = OH: 4-O-(2-amino-2-deoxy-α-d-glucopyranosyl)-2-deoxy-5-O-[3-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-β-d-ribofuranosyl]-d-streptamine (paromomycin II or neomycin F),
G. R1 = H, R2 = CH2-NH2, R3 = CO-CH3, R4 = NH2: 3-N-acetyl-2-deoxy-4-O-(2,6-diamino-2,6-dideoxy-α-d-glucopyranosyl)-5-O-[3-O-(2,6-diamino-2,6-dideoxy-β-l-idopyranosyl)-β-d-ribofuranosyl]-d-streptamine (neomycin B-LP).
Ph Eur

