- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Pemetrexed Disodium Heptahydrate |
 |
(Ph. Eur. monograph 2637)
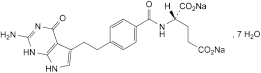
C20H19N5Na2O6,7H2O 597.5 357166-29-1
Thymidylate synthetase inhibitor; cytostatic.
Ph Eur
Disodium (2S)-2-[[4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioate heptahydrate.
97.5 per cent to 102.0 per cent (anhydrous substance).
White or almost white powder.
Freely soluble in water, very slightly soluble in anhydrous ethanol, practically insoluble in methylene chloride.
Carry out either tests A, C, D, E or tests B, C, D, E.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison pemetrexed sodium heptahydrate CRS.
B. Nuclear magnetic resonance spectrometry (2.2.33).
Preparation 25-50 mg/mL solution in deuterium oxide R.
Comparison Solution of equal concentration of pemetrexed disodium heptahydrate CRS in deuterium oxide R.
Results The 1H NMR spectrum obtained is qualitatively similar to the 1H NMR spectrum obtained with pemetrexed disodium heptahydrate CRS; disregard the peak located at approximately 5.0 ppm for the comparison.
C. It gives reaction (a) of sodium (2.3.1).
D. Enantiomeric purity (see Tests).
E. Water (see Tests).
Dissolve 0.56 g in carbon dioxide-free water R and dilute to 10.0 mL with the same solvent.
Solution S is not more opalescent than reference suspension II (2.2.1) and not more intensely coloured than reference solution GY4 or Y4 (2.2.2, Method II).
7.5 to 8.4 for solution S.
Liquid chromatography (2.2.29). Prepare the solutions immediately before use or store them at 2-8 °C for not more than 24 h.
Solution A Dissolve 8 g of β-cyclodextrin R in 900 mL of water for chromatography R. Add 15 mL of triethylamine R then 6 mL of phosphoric acid R and adjust to pH 6.0 with phosphoric acid R. Dilute to 1000 mL with water for chromatography R.
Test solution Dissolve 12 mg of the substance to be examined in water for chromatography R and dilute to 50.0 mL with the same solvent.
Reference solution (a) Dissolve 6 mg of pemetrexed for system suitability CRS (containing impurity E) in water for chromatography R and dilute to 25.0 mL with the same solvent.
Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with water for chromatography R. Dilute 3.0 mL of this solution to 10.0 mL with water for chromatography R.
- — size: l = 0.25 m, Ø = 4.6 mm;
- — stationary phase: octadecylsilyl silica gel for chromatography R (5 µm) with a pore size of 12 nm;
- — temperature: 40 °C.
Mobile phase acetonitrile R, solution A (5:95 V/V).
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 230 nm.
Injection 50 µL.
Run time 1.5 times the retention time of pemetrexed.
Relative retention With reference to pemetrexed (retention time = about 30 min): impurity E = about 0.94.
- — symmetry factor: maximum 2.0 for the principal peak in the chromatogram obtained with reference solution (b);
- — peak-to-valley ratio: minimum 5.0, where Hp = height above the baseline of the peak due to impurity E and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to pemetrexed in the chromatogram obtained with reference solution (a).
- — for impurity E, use the concentration of pemetrexed disodium heptahydrate in reference solution (b).
- — impurity E: maximum 0.3 per cent.
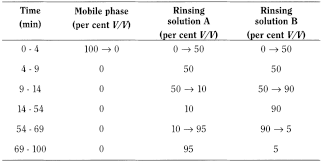
Column rinse The following program is given for information only.
Use a gradient column rinse before column storage or after 30 sample injections to avoid build-up on the column. If a drifting baseline is observed, allow additional time for equilibration with the mobile phase. If a blank chromatogram exhibits broad humps, perform a gradient column rinse.
Rinsing solution A water for chromatography R.
Rinsing solution B acetonitrile R1.
Liquid chromatography (2.2.29). Prepare the solutions immediately before use or store them at 2-8 °C for not more than 24 h.
Solution A 1.45 g/L solution of ammonium formate R in water for chromatography R, adjusted to pH 3.5 with anhydrous formic acid R.
Test solution Dissolve 20 mg of the substance to be examined in water for chromatography R and dilute to 100.0 mL with the same solvent.
Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with water for chromatography R. Dilute 1.0 mL of this solution to 10.0 mL with water for chromatography R.
Reference solution (b) In order to prepare impurities B and C in situ, dissolve 30 mg of the substance to be examined in 10.0 mL of a 4.0 g/L solution of sodium hydroxide R, heat at 70 °C for 40 minutes and allow to cool. Dilute 1.0 mL of the solution to 10.0 mL with water for chromatography R.
Reference solution (c) Dissolve the contents of a vial of pemetrexed impurity mixture CRS (impurities A and D) in 1.0 mL of water for chromatography R.
- — size: l = 0.15 m, Ø = 4.6 mm;
- — stationary phase: base-deactivated octylsilyl silica gel for chromatography R (3.5 µm).
- — mobile phase A: acetonitrile R, solution A (5:95 V/V);
- — mobile phase B: acetonitrile R, solution A (30:70 V/V);

Flow rate 1.0 mL/min.
Detection Spectrophotometer at 250 nm.
Injection 20 µL.
Identification of impurities Use the chromatogram supplied with pemetrexed impurity mixture CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities A and D; use the chromatogram obtained with reference solution (b) to identify the peaks due to impurities B and C.
Relative retention With reference to pemetrexed (retention time = about 26 min): impurity A = about 0.82; impurity B = about 0.87; impurity C = about 0.88; impurity D = about 0.90.
System suitability Reference solution (b):
- — peak-to-valley ratio: minimum 1.5, where Hp = height above the baseline of the peak due to impurity B and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity C.
- — for each impurity, use the concentration of pemetrexed disodium heptahydrate in reference solution (a).
- — impurities A, D: for each impurity, maximum 0.15 per cent;
- — unspecified impurities: for each impurity, maximum 0.10 per cent;
- — total: maximum 0.6 per cent;
- — reporting threshold: 0.05 per cent.
Maximum 20 ppm.
Solvent mixture acetone R, water R (40:60 V/V).
0.250 g complies with test H. Prepare the reference solution using 0.5 mL of lead standard solution (10 ppm Pb) R.
19.5 per cent to 22.1 per cent, determined on 0.050 g.
Less than 0.17 IU/mg.
Liquid chromatography (2.2.29). Prepare the solutions immediately before use or store them at 2-8 °C for not more than 24 h.
Acetate buffer Mix 1.7 mL of glacial acetic acid R and 900 mL of water for chromatography R, adjust to pH 5.3 with a 760 g/L solution of sodium hydroxide R in water for chromatography R and dilute to 1000 mL with water for chromatography R.
Test solution Dissolve 30.0 mg of the substance to be examined in water for chromatography R and dilute to 200.0 mL with the same solvent.
Reference solution Dissolve 30.0 mg of pemetrexed disodium heptahydrate CRS in water for chromatography R and dilute to 200.0 mL with the same solvent.
- — size: l = 0.15 m, Ø = 4.6 mm;
- — stationary phase: base-deactivated octylsilyl silica gel for chromatography R (3.5 µm);
- — temperature: 30 °C.
Mobile phase acetonitrile R, acetate buffer (11:89 V/V).
Flow rate 2.0 mL/min.
Detection Spectrophotometer at 285 nm.
Injection 20 µL.
Run time Twice the retention time of pemetrexed (retention time = about 3 min).
Calculate the percentage content of C20H19N5Na2O6 taking into account the assigned content of pemetrexed disodium heptahydrate CRS.
Specified impurities A, D, E.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use): B, C.
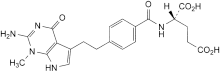
A. (2S)-2-[[4-[2-(2-amino-1-methyl-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]-pentanedioic acid,

B. (2S,2′S)-2,2′-[[(5R)-2,2′-diamino-4,4′,6-trioxo-1,4,4′,6,7,7′-hexahydro-1′H,5H-5,6′-bipyrrolo[2,3-d]pyrimidine-5,5′-diyl]bis(ethylenebenzene-4,1-diylcarbonylimino)]dipentanedioic acid,

C. (2S,2′S)-2,2′-[[(5S)-2,2′-diamino-4,4′,6-trioxo-1,4,4′,6,7,7′-hexahydro-1′H,5H-5,6′-bipyrrolo[2,3-d]pyrimidine-5,5′-diyl]bis(ethylenebenzene-4,1-diylcarbonylimino)]dipentanedioic acid,

D. (2S)-2-[[(4S)-4-[[4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]-4-carboxybutanoyl]amino]pentanedioic acid,

E. (2R)-2-[[4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioic acid.
Ph Eur

