- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Sucrose |
 |
Refined Sugar
(Ph. Eur. monograph 0204)
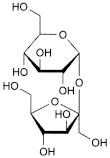
C12H22O11 342.3 57-50-1
Sweetening agent.
Ph Eur
β-d-Fructofuranosyl α-d-glucopyranoside.
It contains no additives.
White or almost white, crystalline powder, or lustrous, colourless or white or almost white crystals.
Very soluble in water, slightly soluble in ethanol (96 per cent), practically insoluble in anhydrous ethanol.
First identification A.
Second identification B, C.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison sucrose CRS.
B. Thin-layer chromatography (2.2.27).
Test solution Dissolve 10 mg of the substance to be examined in a mixture of 2 volumes of water R and 3 volumes of methanol R and dilute to 20 mL with the same mixture of solvents.
Reference solution (a) Dissolve 10 mg of sucrose CRS in a mixture of 2 volumes of water R and 3 volumes of methanol R and dilute to 20 mL with the same mixture of solvents.
Reference solution (b) Dissolve 10 mg each of fructose CRS, glucose CRS, lactose CRS and sucrose CRS in a mixture of 2 volumes of water R and 3 volumes of methanol R and dilute to 20 mL with the same mixture of solvents.
Plate TLC silica gel G plate R.
Mobile phase Cold saturated boric acid solution R, 60 per cent V/V solution of glacial acetic acid R, ethanol R, acetone R, ethyl acetate R (10:15:20:60:60 V/V/V/V/V).
Application 2 µL.
Development In an unsaturated tank over a path of 15 cm.
Drying In a current of warm air.
Detection Spray with a solution of 0.5 g of thymol R in a mixture of 5 mL of sulfuric acid R and 95 mL of alcohol R. Heat the plate at 130 °C for 10 min.
System suitability The chromatogram obtained with reference solution (b) shows 4 clearly separated spots.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with reference solution (a).
C. Dilute 1 mL of solution S (see Tests) to 100 mL with water R. To 5 mL of the solution add 0.15 mL of freshly prepared copper sulfate solution R and 2 mL of freshly prepared dilute sodium hydroxide solution R. The solution is blue and clear and remains so after boiling. To the hot solution add 4 mL of dilute hydrochloric acid R and boil for 1 min. Add 4 mL of dilute sodium hydroxide solution R. An orange precipitate is formed immediately.
Dissolve 50.0 g in water R and dilute to 100 mL with the same solvent.
Solution S is clear (2.2.1).
Maximum 35 µS·cm-1 at 20 °C.
Dissolve 31.3 g in carbon dioxide-free water R prepared from distilled water R and dilute to 100 mL with the same solvent. Measure the conductivity of the solution (C1), while gently stirring with a magnetic stirrer, and that of the water used for preparing the solution (C2). The readings must be stable within 1 per cent over a period of 30 s. Calculate the conductivity of the solution of the substance to be examined from the following expression:
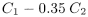
+ 66.3 to + 67.0.
Dissolve 26.0 g in water R and dilute to 100.0 mL with the same solvent.
Maximum 45.
Dissolve 50.0 g in 50.0 mL of water R. Mix, filter (diameter of pores 0.45 µm) and degas. Measure the absorbance (2.2.25) at 420 nm, using a cell of minimum 4 cm (a cell length of 10 cm or more is preferred).
Calculate the colour value using the following expression:
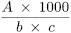
A |
= |
absorbance measured at 420 nm; |
b |
= |
path length in centimetres; |
c |
= |
concentration of the solution, in grams per millilitre, calculated from the refractive index (2.2.6) of the solution; use Table 0204.-1 and interpolate the values if necessary. |
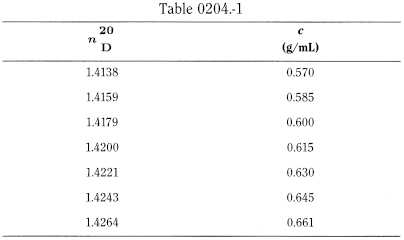
- — repeatability: the absolute difference between 2 results is not greater than 3.
If intended for use in the manufacture of large-volume parenteral preparations, it complies with the test for dextrins. To 2 mL of solution S add 8 mL of water R, 0.05 mL of dilute hydrochloric acid R and 0.05 mL of 0.05 M iodine. The solution remains yellow.
To 5 mL of solution S in a test-tube about 150 mm long and 16 mm in diameter add 5 mL of water R, 1.0 mL of 1 M sodium hydroxide and 1.0 mL of a 1 g/L solution of methylene blue R. Mix and place in a water-bath. After exactly 2 min, take the tube out of the bath and examine the solution immediately. The blue colour does not disappear completely. Ignore any blue colour at the air/solution interface.
Maximum 10 ppm, calculated as SO2.
Determine the sulfites content by a suitable enzymatic method based on the following reactions. Sulfite is oxidised by sulfite oxidase to sulfate and hydrogen peroxide which in turn is reduced by nicotinamide-adenine dinucleotide-peroxidase in the presence of reduced nicotinamide-adenine dinucleotide (NADH). The amount of NADH oxidised is proportional to the amount of sulfite.
Test solution Dissolve 4.0 g of the substance to be examined in freshly prepared distilled water R and dilute to 10.0 mL with the same solvent.
Reference solution Dissolve 4.0 g of the substance to be examined in freshly prepared distilled water R, add 0.5 mL of sulfite standard solution (80 ppm SO2) R and dilute to 10.0 mL with freshly prepared distilled water R.
Blank solution Freshly prepared distilled water R.
Separately introduce 2.0 mL each of the test solution, the reference solution and the blank in 10 mm cuvettes and add the reagents as described in the instructions in the kit for sulfite determination. Measure the absorbance (2.2.25) at the absorption maximum at about 340 nm before and at the end of the reaction time and subtract the value obtained with the blank.
The absorbance difference of the test solution is not greater than half the absorbance difference of the reference solution.
Maximum 0.1 per cent, determined on 2.000 g by drying in an oven at 105 °C for 3 h.
Less than 0.25 IU/mg, if intended for use in the manufacture of large-volume parenteral preparations.
The label states, where applicable, that the substance is suitable for use in the manufacture of large-volume parenteral preparations.
Ph Eur

