- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Acetylcysteine Injection |
Sulfydryl donor; antidote to paracetamol poisoning; mucolytic.
Acetylcysteine Injection is a sterile solution in Water for Injections of acetylcysteine sodium, prepared by the interaction of Acetylcysteine with Sodium Hydroxide.
The injection complies with the requirements stated under Parenteral Preparations and with the following requirements.
95.0 to 105.0% of the stated amount.
To a volume containing the equivalent of 0.8 g of acetylcysteine add 3m hydrochloric acid until the pH of the solution is 2. Add, while stirring continuously, two 200-mg portions of finely powdered sodium chloride followed, if necessary, by further 25-mg portions of sodium chloride until a precipitate begins to appear. Allow to stand for 15 minutes, filter and dry the residue at 70° at a pressure not exceeding 0.7 kPa for 2 hours. The infrared absorption spectrum of the residue, Appendix II A, is concordant with the reference spectrum of acetylcysteine (RS 003). Examine as discs prepared using potassium bromide.
pH, 6.5 to 7.5, Appendix V L.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. With the exception of solution (3), the solutions should be prepared immediately before use.
(1) Dilute the injection with the mobile phase to produce a solution containing the equivalent of 0.2% w/v of Acetylcysteine.
(2) 0.2% w/v solution of N-acetyl-l-cysteine in the mobile phase.
(3) 0.2% w/v solution of N-acetyl-l-cysteine in the mobile phase and store at room temperature for at least 2 hours before use.
(4) Dissolve 20 mg of l-cysteine and 20 mg of l-cystine in 10 mL of 1m hydrochloric acid, add 40 mg of N-acetyl-l-cysteine and immediately dilute to 100 mL with the mobile phase. Dilute 10 mL of the resulting solution to 200 mL with the mobile phase.
(5) Dilute 1 volume of solution (4) to 10 volumes with mobile phase.
(a) Use a stainless steel column (25 cm × 5 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Lichrosorb RP18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 205 nm.
(f) Inject 20 µL of each solution.
(g) Allow the chromatography to proceed for three times the retention time of acetylcysteine.
The retention times of cystine, cysteine and acetylcysteine are about 3.6 minutes, 4 minutes and 6 minutes respectively.
10 volumes of methanol and 90 volumes of a 0.5% w/v solution of ammonium sulfate containing 0.02m sodium pentanesulfonate, the solution being adjusted to pH 2.0 using 2m hydrochloric acid.
The test is not valid unless:
in the chromatogram obtained with solution (4), the height of the trough separating the peaks due to cysteine and cystine is less than one quarter of the height of the peak due to cysteine;
in the chromatogram obtained with solution (3), a peak due to N,N′-diacetylcystine appears which has a retention time of about 13 minutes. The area of this peak is greater than the area of any corresponding peak in the chromatogram obtained with solution (2).
In the chromatogram obtained with solution (1):
the area of any peak corresponding to N,N′-diacetylcystine is not greater than the area of the peak due to acetylcysteine in the chromatogram obtained with solution (4) (1%);
the area of any peak due to cysteine or cystine is not greater than the corresponding peak in the chromatogram obtained with solution (4) (0.5%);
the sum of the areas of any other secondary peaks is not greater than the area of the peak due to acetylcysteine in the chromatogram obtained with solution (4) (1%).
Disregard any peak with an area less than the area of the peak due to acetylcysteine in the chromatogram obtained with solution (5) (0.1%).
Place a quantity of the injection containing the equivalent of 0.4 g of Acetylcysteine in a round-bottomed, three-necked flask containing 40 mL of water. The flask is fitted with a gas inlet tube which reaches nearly to the bottom of the flask, a dropping funnel containing hydrochloric acid and an outlet tube leading to a 100 mL graduated flask containing a mixture of 1 mL of 5m sodium hydroxide and 50 mL of water. Pass through the flask a steady current of nitrogen and add 10 mL of hydrochloric acid from the dropping funnel. Maintain the current of nitrogen for 30 minutes and then disconnect the absorption flask. Add to the flask 10 mL of a solution prepared by dissolving 0.1 g of N,N-dimethyl-p-phenylenediamine dihydrochloride in a mixture of 45 mL of hydrochloric acid and 55 mL of water decolourised with activated charcoal before use, if necessary, and 5 mL of a 5% w/v solution of iron(III) chloride hexahydrate in 1m hydrochloric acid and allow to stand for 20 minutes protected from light. Add sufficient water to produce 100 mL and measure the absorbance of the solution, Appendix II B, at 665 nm using a 4-cm pathlength and using in the reference cell a solution prepared in the same manner but without the injection being examined.
Prepare a 0.4% w/v solution of sodium sulfide. Standardise this solution in the following manner. To 25 mL of 0.05m iodine VS add 8 mL of hydrochloric acid and 25 mL of the sodium sulfide solution. Titrate with 0.1m sodium thiosulfate solution VS using starch solution, added towards the end point, as indicator. Repeat the operation without the sodium sulfide solution. The concentration of the sodium sulfide solution expressed in parts per million of hydrogen sulfide is the difference between the titrations multiplied by 68.16. Prepare a solution containing the equivalent of 20 ppm of hydrogen sulfide by appropriate dilution of the sodium sulfide solution with water.
Repeat the procedure carried out on the injection using 2 mL of the 20-ppm hydrogen sulfide solution in place of the injection being examined. The absorbance of the solution obtained from the injection is not greater than the absorbance of the solution obtained from the standard (100 ppm with reference to the content of acetylcysteine).
Carry out the test for bacterial endotoxins, Appendix XIV C. If necessary, dilute the injection with water BET to give a solution containing 10 mg per mL (solution A). The endotoxin limit concentration of solution A is not more than 0.3 IU per mL.
Add 20 mL of glacial acetic acid to a volume containing the equivalent of 0.4 g of Acetylcysteine and titrate with 0.05m iodine VS until a permanent pale yellow colour is obtained. Each mL of 0.05m iodine VS is equivalent to 16.23 mg of C5H9NO3S.
Acetylcysteine Injection should be protected from light.
The strength is stated in terms of the equivalent amount of Acetylcysteine in a suitable dose-volume.
The impurities limited by the requirements of this monograph include:
1. L-cystine,
2. L-cysteine,
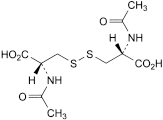
3. N,N′-diacetyl-l-cystine.

