- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Cetirizine Oral Solution |
Histamine H1 receptor antagonist; antihistamine.
Cetirizine Oral Solution is a solution of Cetirizine Hydrochloride in a suitable flavoured vehicle.
The oral solution complies with the requirements stated under Oral Liquids and with the following requirements.
95.0 to 105.0% of the stated amount.
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions.
(1) Dilute a quantity of the oral solution, if necessary, to contain 0.1% w/v of Cetirizine Hydrochloride, filter through a 0.45-µm nylon filter and use the filtrate.
(2) 0.1% w/v of cetirizine hydrochloride BPCRS.
(3) 0.1% w/v of cetirizine hydrochloride BPCRS and 0.1% w/v of chlorphenamine maleate BPCRS.
(a) Use as the coating silica gel F254 (Merck silica gel 60 F254 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 5 µL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in air and examine under ultraviolet light (254 nm).
1 volume of 18m ammonia, 10 volumes of methanol and 90 volumes of dichloromethane.
The test is not valid unless the chromatogram obtained with solution (3) shows two clearly separated spots.
The principal spot in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2).
Disregard any spots due to excipients.
B. In the Assay, the principal peak in the chromatogram obtained with solution (1) has the same retention time as the principal peak in the chromatogram obtained with solution (2).
pH, 4.5 to 5.5, Appendix V L.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in mobile phase A.
(1) Dilute a quantity of the oral solution, if necessary, to contain 0.02% w/v of Cetirizine Hydrochloride and filter through a 0.45-µm nylon filter.
(2) Dilute 1 volume of solution (1) to 100 volumes and dilute 3 volumes of the resulting solution to 10 volumes.
(3) Dilute 1 volume of solution (1) to 100 volumes and dilute 2 volumes of the resulting solution to 10 volumes.
(4) 0.0005% w/v of cetirizine impurity standard BPCRS.
chromatographic conditions
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Phenomenex Luna C18(2) is suitable).
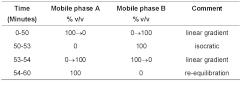
(b) Use gradient elution and the mobile phases described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use a column temperature of 30°.
(e) Use a detection wavelength of 230 nm.
(f) Inject 20 µL of each solution.
mobile phase
Mobile phase A 17 volumes of acetonitrile and 83 volumes of water, adjust the pH to 1.5 with orthophosphoric acid.
Mobile phase B 35 volumes of acetonitrile and 65 volumes of water previously adjusted to pH 1.5 with orthophosphoric acid.
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution factor between the peaks due to cetirizine and cetirizine impurity B is at least 1.5.
In the chromatogram obtained with solution (1):
the areas of any peaks corresponding to impurities A, B or G are not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.3%);
the area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (0.2%);
the total content of impurities is not greater 5 times the area of the principal peak in the chromatogram obtained with solution (3) (1.0%).
Disregard any peak with an area less than 0.25 times the area of the principal peak in the chromatogram obtained with solution (3) (0.05%)
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in mobile phase A.
(1) Dilute a quantity of the oral solution to contain 0.002% w/v of Cetirizine Hydrochloride and filter through a 0.45-µm nylon filter.
(2) 0.002% w/v of cetirizine hydrochloride BPCRS.
(3) 0.0005% w/v of cetirizine impurity standard BPCRS.
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Phenomenex Luna C18(2) is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use a column temperature of 30°.
(e) Use a detection wavelength of 230 nm.
(f) Inject 20 µL of each solution.
mobile phase
30 volumes of acetonitrile and 70 volumes of 0.0025m potassium dihydrogen orthophosphate, previously adjusted to pH 1.5 with orthophosphoric acid.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to cetirizine and cetirizine impurity B is at least 1.5.
Determine the weight per mL of the oral solution, Appendix V G, and calculate the content of C21H25ClN2O3,2HCl, weight in volume, using the declared content of C21H25ClN2O3,2HCl in cetirizine hydrochloride BPCRS.

