- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Cetirizine Tablets |
Histamine H1 receptor antagonist; antihistamine.
Cetirizine Tablets contain Cetirizine Hydrochloride. They are coated.
The tablets comply with the requirements stated under Tablets and with the following requirements.
95.0 to 105.0% of the stated amount.
Shake a quantity of the powdered tablets containing 50 mg of Cetirizine Hydrochloride with 20 mL of absolute ethanol, filter (Whatman GF/C paper is suitable), evaporate the filtrate and dry the residue at 60° for 1 hour. The infrared absorption spectrum of the residue, Appendix II A, is concordant with the reference spectrum of cetirizine hydrochloride (RS 456).
Comply with the dissolution test for tablets and capsules, Appendix XII B1.
(a) Use Apparatus 2, rotating the paddle at 50 revolutions per minute.
(b) Use 900 mL of water, at a temperature of 37°, as the medium.
(1) After 45 minutes withdraw a sample of 10 mL of the medium, filter and dilute the filtrate with sufficient of the dissolution medium to give a solution expected to contain about 0.00050% w/v of Cetirizine Hydrochloride. Measure the absorbance of this solution, Appendix II B, at the maximum wavelength at 230 nm and at 260 nm using the dissolution medium in the reference cell.
(2) Measure the absorbance of a suitable solution of cetirizine BPCRS in water at the maximum wavelength at 230 nm and 260 nm using the dissolution medium in the reference cell and calculate the difference between the two readings (ΔA) for each measurement.
Calculate the total content of Cetirizine hydrochloride, C21H25ClN2O3,2HCl, in the medium using the values of ΔA and from the declared content of C21H25ClN2O3,2HCl in cetirizine hydrochloride BPCRS.
The amount of cetirizine hydrochloride released is not less than 80% (Q) of the stated amount.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in mobile phase A.
(1) Shake a quantity of the powdered tablets containing 20 mg of Cetirizine Hydrochloride with 20 mL of the mobile phase A, filter through a 0.45-µm nylon filter and use the filtrate.
(2) Dilute 1 volume of solution (1) to 100 volumes and dilute 3 volumes of the resulting solution to 10 volumes.
(3) Dilute 1 volume of solution (1) to 100 volumes and dilute 2 volumes of the resulting solution to 10 volumes.
(4) 0.0005% w/v of cetirizine impurity standard BPCRS.
chromatographic conditions
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Phenomenex Luna C18(2) is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use a column temperature of 30°.
(e) Use a detection wavelength of 230 nm.
(f) Inject 20 µL of each solution.
mobile phase
Mobile phase A 17% v/v of acetonitrile and 83% v/v of water, the apparent pH adjusted to 1.5 with orthophosphoric acid.
Mobile phase B 35% v/v of acetonitrile and 65% v/v of water the apparent pH adjusted to 1.5 with orthophosphoric acid.
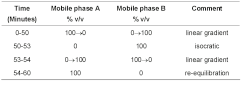
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution factor between the peaks due to cetirizine and impurity B is at least 1.5.
In the chromatogram obtained with solution (1):
the areas of any peaks corresponding to impurities A, B or G are not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.3%);
the area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (0.2%);
the total content of impurities is not greater 5 times the area of the principal peak in the chromatogram obtained with solution (3) (1.0%).
Disregard any peak with an area less than 0.25 times the area of the principal peak in the chromatogram obtained with solution (3) (0.05%)
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions in the mobile phase.
(1) Shake a quantity of the powdered tablets containing 20 mg of Cetirizine Hydrochloride in 100 mL and filter through a 0.45-µm nylon filter. Dilute 10 mL of the filtrate to 100 mL with the mobile phase.
(2) 0.002% w/v of cetirizine hydrochloride BPCRS.
(3) 0.0005% w/v of cetirizine impurity standard BPCRS.
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Phenomenex Luna C18(2) is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use a column temperature of 30°.
(e) Use a detection wavelength of 230 nm.
(f) Inject 20 µL of each solution.
mobile phase
30 volumes of acetonitrile and 70 volumes of 0.0025m potassium dihydrogen orthophosphate, previously adjusted to pH 1.5 with orthophosphoric acid.
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to cetirizine and cetirizine impurity B is at least 1.5.
Calculate the content of C21H25ClN2O3,2HCl in the tablets using the declared content of C21H25ClN2O3,2HCl in cetirizine hydrochloride BPCRS.

