- British Pharmacopoeia Volume IV
- Herbal Drugs, Herbal Drug Preparations and Herbal Medicinal Products
Ginseng Dry Extract |
 |
(Ph. Eur. monograph 2356)
Ph Eur
Dry extract produced from Ginseng (1523).
Minimum 4.0 per cent of the sum of ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1 and Rg2, expressed as ginsenoside Rb1 (C54H92O23; Mr 1109) (dried extract).
The extract is produced from the herbal drug by a suitable procedure using a hydroalcoholic solvent equivalent in strength to ethanol (35-90 per cent V/V).
Light brownish-yellow, hygroscopic powder or brittle mass.
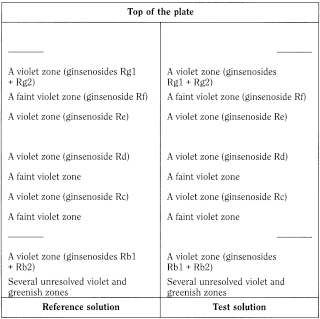
Thin-layer chromatography (2.2.27).
Test solution Dissolve 0.15 g of the extract to be examined in 10 mL of a 70 per cent V/V solution of methanol R.
Reference solution Dissolve 0.15 g of ginseng dry extract HRS in 10 mL of a 70 per cent V/V solution of methanol R.
Plate TLC silica gel plate R (5-40 µm) [or TLC silica gel plate R (2-10 µm)].
Mobile phase ethyl acetate R, water R, butanol R (25:50:100 V/V/V); allow the phases to separate for 10 min and use the upper layer.
Application 20 µL [or 4 µL] as bands of 10 mm [or 8 mm].
Development Over a path of 10 cm [or 5 cm] in an unsaturated tank.
Drying In air.
Detection Treat with anisaldehyde solution R and heat at 105-110 °C for 5-10 min; examine in daylight.
Results See below the sequence of zones present in the chromatograms obtained with the reference solution and the test solution. Furthermore, other faint zones may be present in the chromatograms obtained with the test solution and the reference solution.
Maximum 7.0 per cent.
Liquid chromatography (2.2.29).
Buffer solution Dissolve 3.5 g of disodium hydrogen phosphate dihydrate R and 7.2 g of potassium dihydrogen phosphate R in water R and dilute to 1000 mL with the same solvent.
Test solution Dissolve 0.100 g of the extract to be examined in the buffer solution and dilute to 10.0 mL with the buffer solution. Prepare a ready-to-use sample-preparation cartridge containing 0.50 g of octadecylsilyl silica gel (45 µm), using 5 mL of methanol R followed by 20 mL of water R. Apply 5.0 mL of the solution to be analysed to the top of the cartridge. Wash the cartridge with 20 mL of water R followed by 15 mL of a 30 per cent V/V solution of methanol R. Discard the eluates after confirming that no ginsenosides are present, otherwise repeat the preparation of the solution with another brand of cartridge where no ginsenosides are eluted with a 30 per cent V/V solution of methanol R. Elute the cartridge with 20 mL of methanol R; collect the eluate. Under reduced pressure, evaporate the eluate to dryness. Dissolve the residue in 2.0 mL of methanol R. Filter through a suitable membrane filter (nominal pore size 0.45 µm).
Reference solution (a) Dissolve 0.100 g of ginseng dry extract HRS in the buffer solution and dilute to 10.0 mL with the buffer solution. Prepare a ready-to-use sample-preparation cartridge containing 0.50 g of octadecylsilyl silica gel (45 µm), using 5 mL of methanol R followed by 20 mL of water R. Apply 5.0 mL of the solution to be analysed to the top of the cartridge. Wash the cartridge with 20 mL of water R followed by 15 mL of a 30 per cent V/V solution of methanol R. Discard the eluates after confirming that no ginsenosides are present, otherwise repeat the preparation of the solution with another brand of cartridge where no ginsenosides are eluted with a 30 per cent V/V solution of methanol R. Elute the cartridge with 20 mL of methanol R; collect the eluate. Under reduced pressure, evaporate the eluate to dryness. Dissolve the residue in 2.0 mL of methanol R. Filter through a suitable membrane filter (nominal pore size 0.45 µm).
Reference solution (b) Dissolve 3.0 mg of ginsenoside Rb1 CRS in methanol R and dilute to 5.0 mL with the same solvent.
Reference solution (c) Dissolve 3.0 mg of ginsenoside Rg2 R in methanol R and dilute to 5.0 mL with the same solvent.
Reference solution (d) Dilute 1.0 mL of reference solution (b) to 2.0 mL with reference solution (c).
- — size: l = 0.125 m, Ø = 4.6 mm;
- — stationary phase: octadecylsilyl silica gel for chromatography R (5 µm);
- — temperature: 35 °C.
- — mobile phase A: water R adjusted to pH 2 with phosphoric acid R;
- — mobile phase B: acetonitrile R1;
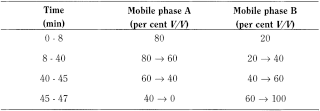
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 203 nm.
Injection 20 µL.
Elution order Ginsenoside Rg1, ginsenoside Re, ginsenoside Rf, ginsenoside Rb1, ginsenoside Rg2, ginsenoside Rc, ginsenoside Rb2, ginsenoside Rd; depending on the operating conditions and the state of the column, ginsenoside Rb1 may elute before or after ginsenoside Rg2.
Identification of peaks Use the chromatogram supplied with ginseng dry extract HRS and the chromatogram obtained with reference solution (a) to identify the peaks due to ginsenosides Rg1, Re, Rf, Rc, Rb2 and Rd; use the chromatogram obtained with reference solution (b) to identify the peak due to ginsenoside Rb1; use the chromatogram obtained with reference solution (c) to identify the peak due to ginsenoside Rg2.
Relative retention With reference to ginsenoside Rb1 (retention time = about 33 min): ginsenoside Rg1 = about 0.53; ginsenoside Re = about 0.54; ginsenoside Rf = about 0.88; ginsenoside Rg2 = about 0.98; ginsenoside Rc = about 1.04; ginsenoside Rb2 = about 1.08; ginsenoside Rd = about 1.17.
System suitability Reference solution (d):
- — resolution: minimum 1.5 between the peaks due to ginsenosides Rg2 and Rb1.
Calculate the percentage content of the sum of ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1 and Rg2, expressed as ginsenoside Rb1, using the following expression:
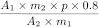
A 1 |
= |
sum of the areas of the peaks due to ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1 and Rg2 in the chromatogram obtained with the test solution; |
A 2 |
= |
area of the peak due to ginsenoside Rb1 in the chromatogram obtained with reference solution (b); |
m 1 |
= |
mass of the extract to be examined used to prepare the test solution, in grams; |
m 2 |
= |
mass of ginsenoside Rb1 CRS used to prepare reference solution (b), in grams; |
p |
= |
percentage content of ginsenoside Rb1 in ginsenoside Rb1 CRS. |
Ph Eur

